Global Dolutegravir Market Size, Share, and COVID-19 Impact Analysis, By Type (10mg Tablets and 50mg Tablets), By Application (Prevent HIV Infection Following Potential Exposure, Treatment of HIV Infection, and Pediatric Use), By End-user (Hospitals, Clinics, Home care, and Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 - 2035
Industry: HealthcareGlobal Dolutegravir Market Size Insights Forecasts to 2035
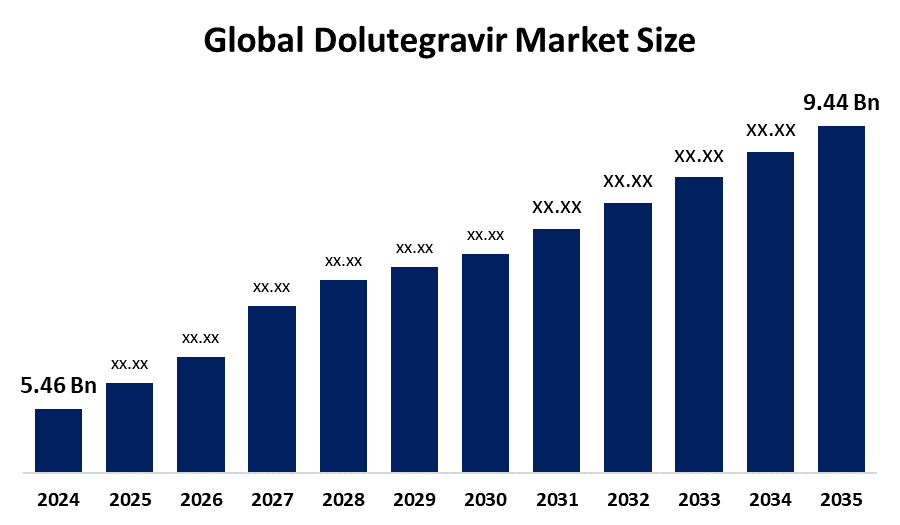
- The Global Dolutegravir Market Size Was Estimated at USD 5.46 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of around 5.1% from 2025 to 2035
- The Worldwide Dolutegravir Market Size is Expected to Reach USD 9.44 Billion by 2035
- Asia Pacific is expected to Grow the fastest during the forecast period.
Get more details on this report -
According to a Research Report Published by Spherical Insights and Consulting, The Global Dolutegravir Market Size was worth around USD 5.46 Billion in 2024 and is predicted to Grow to around USD 9.44 Billion by 2035 with a compound annual growth rate (CAGR) of 5.1% from 2025 to 2035. The global dolutegravir market is growing because the drug delivers high success rates as the first-choice treatment for HIV, and its daily dosing schedule produces better patient compliance, and its generic versions become more accessible in developing countries, and worldwide organizations work to enhance access to HIV treatment.
Market Overview
Dolutegravir (DTG) serves as an efficient second-generation HIV-1 integrase strand transfer inhibitor (INSTI), which doctors use together with various antiretroviral drugs to treat AIDS. The drug operates as a fundamental medicine for primary HIV treatment together with pre-exposure prophylaxis (PrEP) because it stops viral propagation. Market growth is driven by World Health Organisation (WHO) recommendations for DTG-based regimens (such as TLD), which enable effective HIV-1 treatment with high resistance protection and good safety.
On 27 June 2024, Azerbaijan, Belarus, and Kazakhstan achieved major developments in the implementation of dolutegravir-based HIV treatment following the establishment of the Medicines Patent Pool ViiV Healthcare license. Price reductions that exceeded 90% enabled procurement to cover 78% of patients in Belarus, and 86% of patients in Kazakhstan and 65% of patients in Azerbaijan, while governments, communities and global partners worked together to improve access to antiretroviral therapy. The rapid implementation of pediatric medicine formulas for children, together with increased usage in low and middle-income countries that receive international funding and the creation of two-drug treatment methods, which decrease the number of medicines to take, will create new business possibilities. The main market forces include ViiV Healthcare, Aurobindo Pharma, Mylan N.V., Sun Pharmaceutical Industries and Adcock Ingram, which develop low-cost generic medicines for emerging markets in Africa.
Report Coverage
This research report categorizes the dolutegravir market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the dolutegravir market. Recent market developments and competitive strategies, such as expansion, type launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the dolutegravir market.
Global Dolutegravir Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 5.46 Billion |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 5.1% |
| 2035 Value Projection: | USD 9.44 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 265 |
| Tables, Charts & Figures: | 131 |
| Segments covered: | By Type, By Application and COVID-19 Impact Analysis |
| Companies covered:: | Aurobindo Pharma, ViiV Healthcare, Mylan N.V., Adcock Ingram Limited, Sun Pharmaceutical Industries, Aurobindo Pharma, Cipla Ltd., Laurus Labs, Emcure Pharmaceuticals, Hetero Labs Limited, Desano Inc., Zydus Lifesciences Limited, and Others |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The global dolutegravir market exists because the WHO recommends this medication as the first-line HIV treatment according to its proven effectiveness and strong resistance prevention capabilities. The market growth acceleration depends on two key drivers, which include fixed-dose single-tablet regimens TLD that deliver better patient adherence results. The market experiences significant growth because low- and middle-income countries, especially Africa, use large-scale procurement programs which allow them to access affordable generic drugs through their voluntary licensing agreements. The demand for healthcare services rises because more people get diagnosed with HIV, while healthcare spending increases and people become aware of better treatment options that offer improved side effect management than traditional methods.
Restraining Factors
The global dolutegravir market faces three main obstacles, which include drug resistance development among certain patients, weight gain, neurological side effects and strong competition from new advanced antiviral medications. The market faces challenges in particular areas because of inadequate healthcare facilities, combined with insufficient diagnostic capabilities and existing regulatory obstacles.
Market Segmentation
The dolutegravir market share is classified into type, application, and end-user.
- The 50mg tablets segment dominated the market in 2024, approximately 70% and is projected to grow at a substantial CAGR during the forecast period.
Based on the type, the dolutegravir market is divided into 10mg tablets and 50mg tablets. Among these, the 50mg tablets segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period. The 50 mg dolutegravir tablets segment dominated market growth because primary adult treatment and fixed-dose combination regimens, which contain TLD (tenofovir, lamivudine, dolutegravir), received WHO endorsement. Its adoption in national HIV programs, together with its increased patient base and its greater appeal than pediatric 10 mg tablets, drove strong demand, which established it as the main factor for market growth.
- The treatment of HIV infection segment accounted for the largest share in 2024, approximately 75% and is anticipated to grow at a significant CAGR during the forecast period.
Based on the application, the dolutegravir market is divided into prevent HIV infection following potential exposure, treatment of HIV infection, and pediatric use. Among these, the treatment of HIV infection segment accounted for the largest share in 2024 and is anticipated to grow at a significant CAGR during the forecast period. The treatment of HIV infection segment market growth is due to the increasing prevalence of HIV/AIDS globally and strong adoption of dolutegravir-based regimens as first-line and subsequent therapy. The market expansion for therapeutic use developed through government programs and WHO recommendations, which increased ART access for low- and middle-income countries.
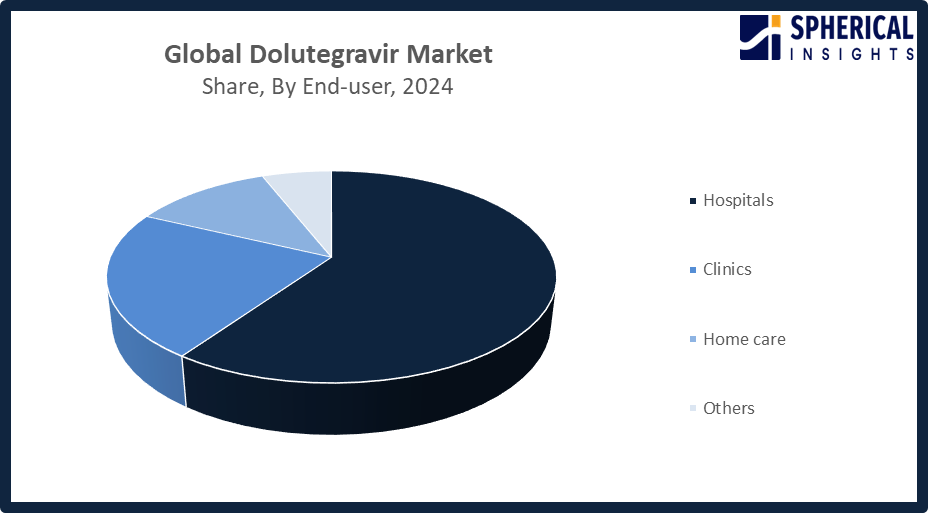
- The hospitals segment accounted for the highest market revenue in 2024, approximately 60% and is anticipated to grow at a significant CAGR during the forecast period.
Based on the end-user, the dolutegravir market is divided into hospitals, clinics, home care, and others. Among these, the hospitals segment accounted for the highest market revenue in 2024 and is anticipated to grow at a significant CAGR during the forecast period. The hospitals segment market growth is due to hospitals functioning as the main centers for starting and handling dolutegravir-based HIV treatment. Hospitals provide comprehensive care, which includes diagnostics and monitoring and complex therapy administration while serving large patient volumes. The government-supported HIV programs, together with structured ART initiatives, established hospitals as the main distribution centres, which enabled the adoption of dolutegravir.
Get more details on this report -
Regional Segment Analysis of the Dolutegravir Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
North America is anticipated to hold the largest share of the dolutegravir market over the predicted timeframe.

Get more details on this report -
North America is anticipated to hold the largest share of the dolutegravir market over the predicted timeframe. North America is expected to hold the 40% share of the dolutegravir market because it has developed healthcare facilities, and people understand HIV, and they use the WHO guidelines for treatment. The United States drives growth through government-supported HIV programs, and its citizens have access to antiretroviral medication, while most patients follow their treatment, and the country invests in research and development and provides both branded and generic medical products. Colombia established its first mandatory generic dolutegravir license in April 2024 to decrease expenses and enhance product availability. Health organizations supported the decision because it enhanced treatment tolerance and decreased treatment resistance while it followed the WHO first-line HIV treatment guidelines.
Asia Pacific is expected to grow at a rapid CAGR in the dolutegravir market during the forecast period. The dolutegravir market in the Asia Pacific will experience a 23% share of rapid growth due to rising HIV cases and expanding antiretroviral therapy programs, and increased access to low-cost generic dolutegravir. The regional growth in India results from government programs, the implementation of ART and the creation of treatment centers through public and private partnerships. The region experiences market growth through rising awareness, better healthcare facilities and the use of dolutegravir-based treatments recommended by the WHO.
The dolutegravir market in Europe experiences constant expansion because people become more aware of HIV, the region has developed healthcare facilities, and people use dolutegravir treatments recommended by the WHO. Germany leads the region through its government-funded HIV treatment initiatives, which provide broad access to both branded and generic dolutegravir to patients who require ART services. The European market expands through its advanced diagnostic systems, which enable early patient treatment and its comprehensive reimbursement models.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the dolutegravir market, along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Aurobindo Pharma
- ViiV Healthcare
- Mylan N.V.
- Adcock Ingram Limited
- Sun Pharmaceutical Industries
- Aurobindo Pharma
- Cipla Ltd.
- Laurus Labs
- Emcure Pharmaceuticals
- Hetero Labs Limited
- Desano Inc.
- Zydus Lifesciences Limited
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In October 2025, ViiV Healthcare announced 96-week PASO DOBLE results showing Dovato (dolutegravir/lamivudine) is as effective as Biktarvy in maintaining HIV-1 viral suppression. Dovato users experienced significantly less weight gain and fewer adverse events. Findings will be presented at the EACS 2025 congress in Paris.
- In July 2024, ViiV Healthcare announced 48-week results from the PASO DOBLE trial, comparing the two-drug regimen Dovato (dolutegravir/lamivudine) with the three-drug regimen Biktarvy in virologically suppressed HIV-1 patients. The study supports Dovato as an effective option for treatment optimization.
- In September 2023, Viatris announced U.S. FDA tentative approval for a pediatric HIV treatment combining abacavir, dolutegravir, and lamivudine as oral suspension tablets. WHO recommends this regimen as first-line therapy. The approval addresses gaps in pediatric HIV care, where 43% of children living with HIV lacked treatment in 2022, contributing to 13% of AIDS-related deaths.
- In September 2023, Strides Pharma announced that its subsidiary, Strides Pharma Global, received USFDA tentative approval for Dolutegravir 50 mg tablets, bioequivalent to ViiV Healthcare’s Tivicay. Manufactured in Bengaluru, the product targets a U.S. market opportunity of $1,345 million under PEPFAR, pending full approval after patent expiry.
- In August 2023, Aurobindo Pharma launched a pediatric HIV triple-combination tablet (Abacavir/Lamivudine/Dolutegravir) under a voluntary license from ViiV Healthcare. Approved tentatively by the USFDA, it targets children in 123 low- and middle-income countries, including India, aiming to improve treatment access and support for young HIV patients.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the dolutegravir market based on the below-mentioned segments:
Global Dolutegravir Market, By Type
- 10mg Tablets
- 50mg Tablets
Global Dolutegravir Market, By Application
- Prevent HIV Infection Following Potential Exposure
- Treatment of HIV Infection
- Pediatric Use
Global Dolutegravir Market, By End-user
- Hospitals
- Clinics
- Home care
- Others
Global Dolutegravir Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the dolutegravir market over the forecast period?The global dolutegravir market is projected to expand at a CAGR of 5.1% during the forecast period.
-
2. What is the market size of the dolutegravir market?The global dolutegravir market size is expected to grow from USD 5.46 billion in 2024 to USD 9.44 billion by 2035, at a CAGR of 5.1% during the forecast period 2025-2035.
-
3. Which region holds the largest share of the dolutegravir market?North America is anticipated to hold the largest share of the dolutegravir market over the predicted timeframe.
-
4. What is the dolutegravir market?The dolutegravir market refers to the global industry for manufacturing, distributing, and selling dolutegravir, an HIV treatment drug.
-
5. Who are the top 10 companies operating in the global dolutegravir market?Aurobindo Pharma, ViiV Healthcare, Mylan N.V., Adcock Ingram Limited, Sun Pharmaceutical Industries, Aurobindo Pharma, Cipla Ltd., Laurus Labs, Emcure Pharmaceuticals, Hetero Labs Limited, and Others.
-
6. What factors are driving the growth of the dolutegravir market?Key drivers include WHO recommendations as preferred first-line HIV treatment, high efficacy with low resistance, widespread generic adoption in low-income regions, and increased, affordable access to single-tablet regimens (TLD).
-
7. What are the market trends in the dolutegravir market?Key trends include rapid adoption of generic TLDs in LMICs, rising demand for pediatric formulations, and a shift toward two-drug regimens.
-
8. What are the main challenges restricting wider adoption of the dolutegravir market?The challenges limiting wider dolutegravir (DTG) adoption include rising drug resistance in patients with treatment experience, adverse effects like weight gain/neural tube defects, logistical supply constraints, and high cost in non-licensed regions.
Need help to buy this report?