Global Prophylactic HIV Drugs Market Size, Share, and COVID-19 Impact Analysis, By Drug (Tenofovir Disoproxil Fumarate, Tenofovir Alafenamide, Emtricitabine, and More), By Dosage Form (Oral Pill, Long-Acting Injectable, and More), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and More), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 - 2035.
Industry: HealthcareGlobal Prophylactic HIV Drugs Market Insights Forecasts to 2035
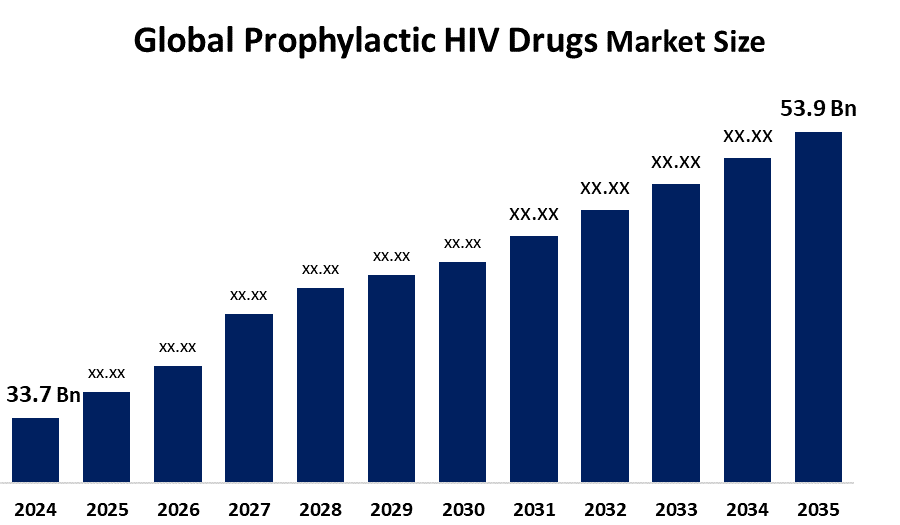
- The Global Prophylactic HIV Drugs Market Size Was Estimated at USD 33.7 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of around 4.36% from 2025 to 2035
- The Worldwide Prophylactic HIV Drugs Market Size is Expected to Reach USD 53.9 Billion by 2035
- Asia Pacific is expected to grow the fastest during the forecast period.
Get more details on this report -
According to a research report published by Spherical Insights and Consulting, The Global Prophylactic HIV Drugs Market Size Was Worth Around USD 33.7 Billion In 2024 And Is Predicted To Grow To Around USD 53.9 Billion By 2035 With A Compound Annual Growth Rate (CAGR) of 4.36% from 2025 to 2035. The global market for prophylactic HIV drugs is growing with the rising number of HIV infections, increased awareness of prevention methods, government interventions, growing accessibility for pre-exposure prophylactic treatments, and advancements in drug development.
Market Overview
The Global Market For Prophylactic HIV Drugs involves medicines for preventing HIV infections among high-risk groups, which are generally used by pre-exposure prevention or other means of prevention. The need for these medications is imperative in the prevention of HIV infections and the regulation of the prevalence of the virus among high-risk groups, including healthcare professionals, persons with behaviour-related risks, and persons living in prevalence areas of HIV. The market for prophylactic HIV medications is growing owing to increased HIV prevalence rates across the globe and increased awareness of preventive healthcare practices.
The WHO in June 2025 welcomed FDA approval for injectable lenacapavir for HIV prevention. Promising trial results in 2024 have placed lenacapavir, given every two years, as one offering sustained protection and a useful addition to current options for pre-exposure prophylaxis for HIV prevention in light of issues with once-daily pills and regular clinic visits. The availability of growth opportunities is further facilitated by innovations within the field of drug formulation: long-acting injectables, combination therapies, and improved delivery mechanisms enhance patient adherence. Increasing access in developing regions and reducing stigma around HIV prevention further support market growth. Major companies active in the market include Gilead Sciences, ViiV Healthcare, Mylan, and Cipla; the companies are reinforcing research and distribution with an eye toward upping their share in growing demands for the preventive treatment against HIV.
Report Coverage
This research report categorizes the Prophylactic HIV Drugs Market Size based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the prophylactic HIV drugs market. Recent market developments and competitive strategies, such as expansion, type launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the prophylactic HIV drugs market.
Global Prophylactic HIV Drugs Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 33.7 Billion |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR of 4.36% |
| 2035 Value Projection: | USD 53.9 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 240 |
| Tables, Charts & Figures: | 90 |
| Segments covered: | By Product Type, By Application |
| Companies covered:: | Gilead Sciences Roche Cipla Ltd. Merck & Co. Mylan Johnson & Johnson ViiV Healthcare Apotex Inc. Hetero Healthcare AbbVie Boehringer Ingelheim Aurobindo Pharma Limited Bristol-Myers Squibb Teva Pharmaceutical Industries Ltd. And Others key players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The rise in cases of HIV/AIDS infection around the world, together with the rise in awareness among people regarding preventive healthcare practices, is the primary factor driving the prophylactic HIV market. The government, along with the World Health Organization, UNAIDS, and other bodies, is actively taking steps forward for the discovery of preventive methods such as Pre-Exposure Prophylaxis, PrEP. Innovations in the development of drugs, especially long-acting injectables, have significantly improved the practicability and success of these drugs. Moreover, the rising adoption of preventative practices in developing countries and a reduced stigma surrounding HIV testing and prevention will be significant factors in the growth of this market.
Restraining Factors
The restraints on the Global Prophylactic HIV Drugs Market Size are the costliness of treatment, lack of accessibility in poor countries, and the tough regimen to follow daily. Other deterrents that stall the growth of the market include side effects, potential issues with drug resistance, and social stigma of HIV prevention.
Market Segmentation
The prophylactic HIV drugs market share is classified into drug, dosage form, and distribution channel.
- The tenofovir alafenamide segment dominated the market in 2024, approximately 55% and is projected to grow at a substantial CAGR during the forecast period.
Based on the drug, the Prophylactic HIV Drugs Market Size is divided into tenofovir disoproxil fumarate, tenofovir alafenamide, emtricitabine, and more. Among these, the tenofovir alafenamide segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period. The tenofovir alafenamide segment is expected to grow due to its enhanced safety profile, reduced renal and bone toxicities, and very effective nature in the prevention of HIV infections. The drug has a favorable safety profile, and its effectiveness and tolerability contributed to its adoption by doctors and patients alike in the healthcare sector of developing nations.
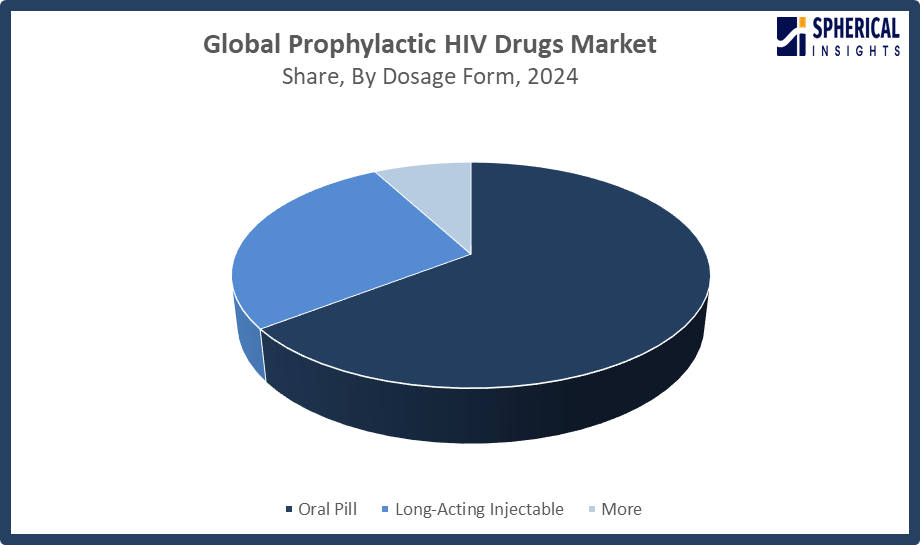
- The oral pill segment accounted for the largest share in 2024, approximately 65% and is anticipated to grow at a significant CAGR during the forecast period.
Based on the dosage form, the prophylactic HIV drugs market is divided into oral pill, long-acting injectable, and more. Among these, the oral pill segment accounted for the largest share in 2024 and is anticipated to grow at a significant CAGR during the forecast period. The oral pills contributed to the market growth as they are proven to be very effective with easy usage. The availability of clear guidelines for prescriptions from healthcare providers, with costs also being less as opposed to injectables, contributed to a higher adoption rate. Additionally, adherence rates were also increased due to familiarity among healthcare providers as well as users.
Get more details on this report -
- The hospital pharmacies segment accounted for the highest market revenue in 2024, approximately 54% and is anticipated to grow at a significant CAGR during the forecast period.
Based on the distribution channel, the Prophylactic HIV Drugs Market Size is divided into hospital pharmacies, retail pharmacies, and more. Among these, the hospital pharmacies segment accounted for the highest market revenue in 2024 and is anticipated to grow at a significant CAGR during the forecast period. The hospital pharmacies distribution channel market growth in the prophylactic treatment of HIV infections is driven by the widespread accessibility of anti-HIV drugs in prophylactic treatment programs initiated in hospital settings. The heavy presence of physicians in these settings and government programs initiated from a public health-care perspective helped boost patient confidence in accessibility and adherence.
Regional Segment Analysis of the Prophylactic HIV Drugs Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
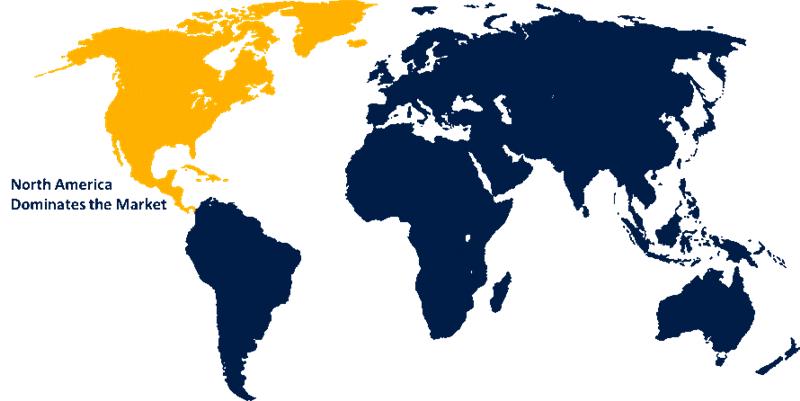
North America is anticipated to hold the largest share of the prophylactic HIV drugs market over the predicted timeframe.
North America is anticipated to hold the largest share of the Prophylactic HIV Drugs Market Size over the predicted timeframe. North America is expected to have a 41% market share of the prophylactic HIV drugs market, while the other regions follow, due to strong healthcare infrastructure, high awareness of the prevention of HIV, and the early adoption of PrEP therapies. The United States leads in this respect, driven by several initiatives such as the PEPFAR, CDC-backed programs for PrEP, and rapid approval of advanced therapies, including long-acting injectables. Canada's contribution through publicly funded healthcare systems aids in bringing about an increase in PrEP accessibility and prevailing preventive HIV screening throughout the country. In September 2025, UNAIDS hailed the U.S. for reaffirming leadership in the AIDS response through PEPFAR. The new strategy puts a strategic emphasis on global HIV targets, country partnerships, and resilient health systems, and pointedly underlines national self-reliance as being at the center of ending AIDS as a public health threat.
Get more details on this report -
Asia Pacific is expected to grow at a rapid CAGR in the prophylactic HIV drugs market during the forecast period. The Asia Pacific market is expected to have 22% market share due to increased HIV awareness, better access to healthcare facilities, and effective government preventive programs. The Asian nations, including India, China, and Thailand, are some of the leading contributors to this market. India is increasing its use of PrEP through healthcare programs, China is emphasizing preventive measures regarding HIV screening, and Thailand is at the forefront of PrEP distribution.
Europe’s PI HIV medicines market is encouraged by the adoption of robust public health care systems, early adoption of PrEP, and governmental initiatives to control the spread of HIV in the region. Countries such as the UK, France, and Germany top the European region. This is because the UK has rolled out PrEP in their public health care systems, while the French were among the first to finance PrEP in their markets. Germany is encouraging PrEP access through insurance coverage and HIV campaigns.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the prophylactic HIV drugs market, along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Gilead Sciences
- Roche
- Cipla Ltd.
- Merck & Co.
- Mylan
- Johnson & Johnson
- ViiV Healthcare
- Apotex Inc.
- Hetero Healthcare
- AbbVie
- Boehringer Ingelheim
- Aurobindo Pharma Limited
- Bristol-Myers Squibb
- Teva Pharmaceutical Industries Ltd.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In October 2025, ViiV Healthcare announced that NICE issued positive guidance recommending its long-acting HIV prevention treatment, Apretude (cabotegravir), for eligible patients in England and Wales. The treatment, manufactured at GSK’s County Durham facility, highlights ViiV’s focus on innovative HIV therapies since its 2009 founding.
- In September 2025, Merck & Co. revealed MK-8527, a once-monthly oral HIV PrEP candidate now in Phase 3 trials. Designed to prevent HIV infection, it offers an alternative to daily pills like Truvada and Descovy, or long-acting injections such as Apretude and Yeztugo.
- In July 2025, Aurobindo Pharma, Cipla, and Viatris announced plans to develop and supply long-acting injectable cabotegravir (CAB LA) for HIV treatment across 133 low- and middle-income countries, aiming to expand global access to advanced HIV therapies.
- In June 2025, Gilead Sciences announced FDA approval of Yeztugo (lenacapavir) as the first twice-yearly injectable PrEP in the U.S. Clinical trials showed ≥99.9% of participants remained HIV-negative, providing a long-acting, highly effective option for adults and adolescents at risk of sexually acquired HIV.
- In February 2025, Gilead Sciences announced FDA acceptance of its New Drug Applications for lenacapavir, a twice-yearly injectable HIV-1 capsid inhibitor for PrEP. Granted Breakthrough Therapy Designation in 2024, the drug is under priority review with a PDUFA target action date set for June 19, 2025.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the prophylactic HIV drugs market based on the below-mentioned segments:
Global Prophylactic HIV Drugs Market, By Drug
- Tenofovir Disoproxil Fumarate
- Tenofovir Alafenamide
- Emtricitabine
- More
Global Prophylactic HIV Drugs Market, By Dosage Form
- Oral Pill
- Long-Acting Injectable
- More
Global Prophylactic HIV Drugs Market, By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- More
Global Prophylactic HIV Drugs Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the prophylactic HIV drugs market over the forecast period?The global prophylactic HIV drugs market is projected to expand at a CAGR of 4.36% during the forecast period.
-
2. What is the prophylactic HIV drugs market?The prophylactic HIV drugs market involves medicines used to prevent HIV infection, primarily through pre-exposure prophylaxis in high-risk populations
-
3. What is the market size of the prophylactic HIV drugs market?The global prophylactic HIV drugs market size is expected to grow from USD 33.7 billion in 2024 to USD 53.9 billion by 2035, at a CAGR of 4.36% during the forecast period 2025-2035
-
4. Which region holds the largest share of the prophylactic HIV drugs market?North America is anticipated to hold the largest share of the prophylactic HIV drugs market over the predicted timeframe
-
5. Who are the top 10 companies operating in the global prophylactic HIV drugs market?Gilead Sciences, Roche, Cipla Ltd., Merck & Co., Mylan, Johnson & Johnson, ViiV Healthcare, Apotex Inc., Hetero Healthcare, AbbVie, Boehringer Ingelheim, and Others.
-
6. What factors are driving the growth of the prophylactic HIV drugs market?The prophylactic HIV drugs market is driven by rising HIV prevalence, increased awareness of preventive healthcare, government initiatives, expanded access to PrEP, advancements in long-acting formulations, and growing global healthcare investments
-
7. What are the market trends in the prophylactic HIV drugs market?Key trends in the prophylactic HIV drugs market include growing adoption of long-acting injectable PrEP options, expansion of generic, affordable drugs, increased emphasis on diverse HIV prevention tools, and stronger regulatory support for innovative formulations.
-
8. What are the main challenges restricting wider adoption of the prophylactic HIV drugs market?The main challenges restricting the wider adoption of prophylactic HIV drugs (PrEP) are stigma, lack of awareness and education, financial and access barriers, and healthcare system biases and limitations. These factors interact to limit uptake, particularly among the populations most at risk for HIV.
Need help to buy this report?