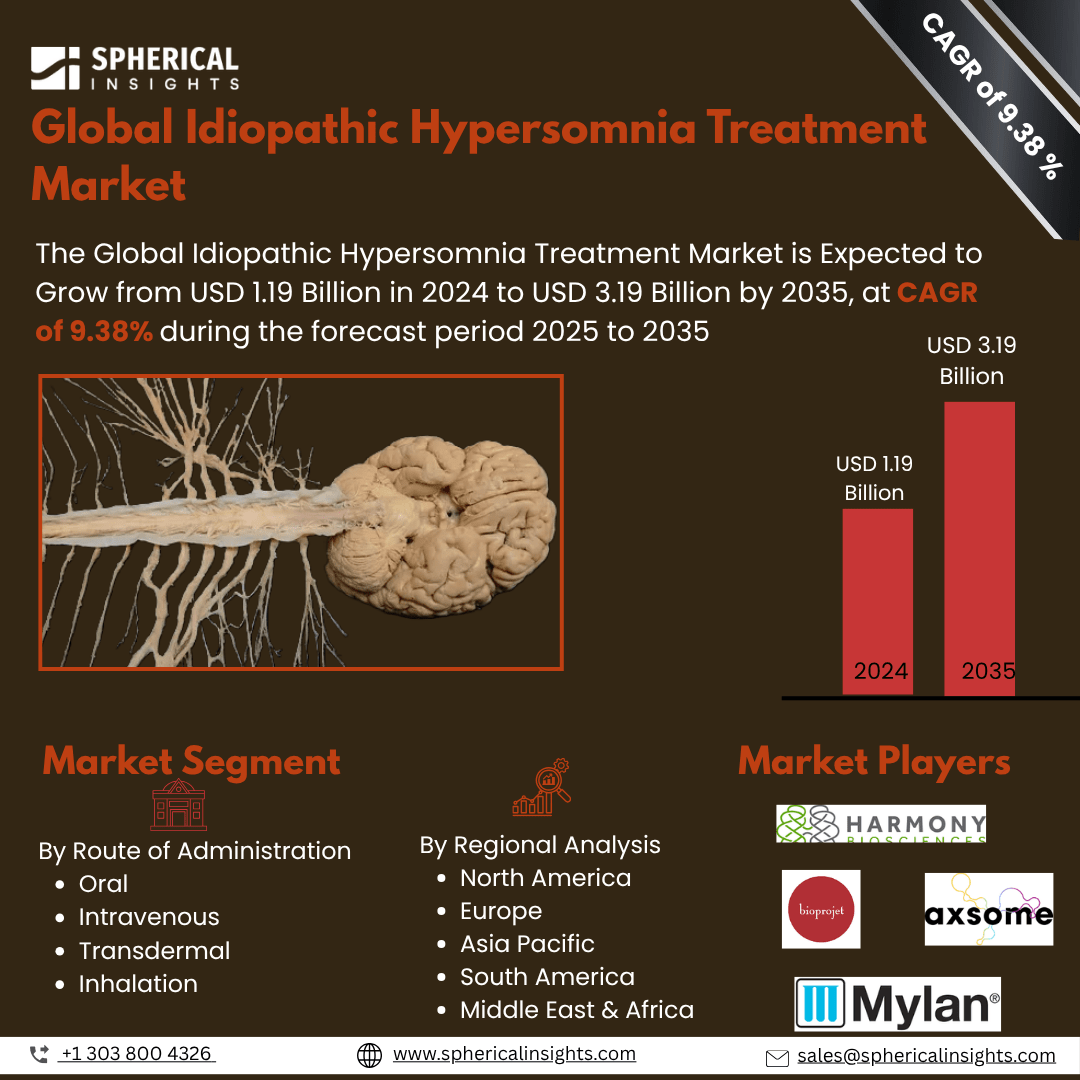
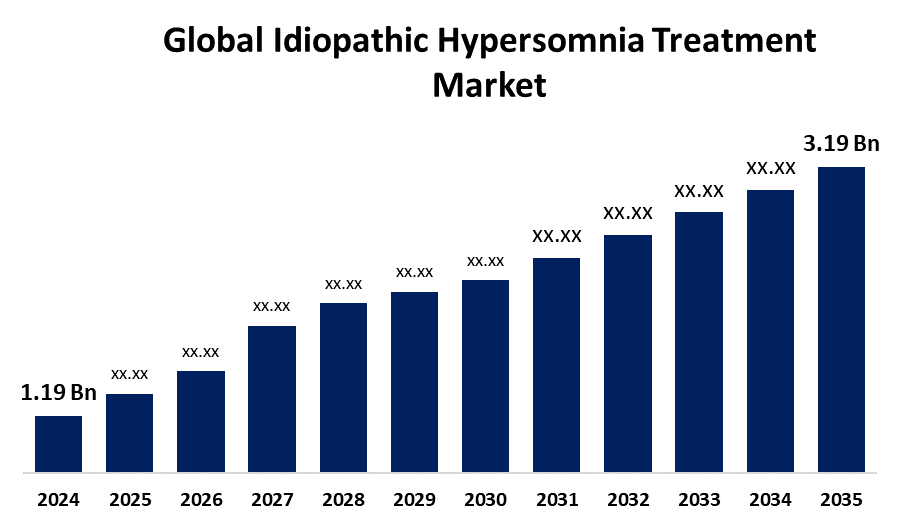
- As per Spherical Insights & Consulting, The Global Idiopathic Hypersomnia Treatment Market is Expected to Grow from USD 1.19 Billion in 2024 to USD 3.19 Billion by 2035, at a CAGR of 9.38% during the forecast period 2025 to 2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Idiopathic Hypersomnia Treatment Market Companies such as Pfizer Inc., Harmony Biosciences, Sun Pharmaceutical Industries, Avadel Pharmaceuticals, Eli Lilly and Company, Jazz Pharmaceuticals, Johnson & Johnson, Teva Pharmaceuticals, Bioprojet, Merck & Co., Takeda Pharmaceutical Company, Mylan N.V., Axsome Therapeutics, Novartis AG, NLS Pharmaceutics, and Others.
Idiopathic Hypersomnia Treatment Market: Understanding and Treatment Algorithm:
Idiopathic Hypersomnia is a chronic sleep disorder marked by excessive daytime sleepiness despite adequate or prolonged nighttime sleep. Unlike narcolepsy, it lacks sudden sleep attacks or cataplexy. Its cause is unknown (“idiopathic”). People with this condition often struggle to wake up and may feel unrefreshed even after long sleep durations.
Idiopathic Hypersomnia Diagnosis:
Diagnosis of Idiopathic Hypersomnia involves ruling out other sleep disorders through clinical evaluation, sleep history, and tests like polysomnography (overnight sleep study) and the Multiple Sleep Latency Test (MSLT). These tests assess sleep duration and daytime sleepiness. A diagnosis is confirmed when excessive sleepiness is unexplained by other medical or psychiatric conditions.
Idiopathic Hypersomnia Treatment:
Treatment for Idiopathic Hypersomnia focuses on managing symptoms, as there is no known cure. Stimulant or wakefulness-promoting medications like modafinil may be prescribed. Good sleep hygiene, scheduled naps, and lifestyle adjustments can help improve daily functioning. Regular follow-up with a sleep specialist is important to monitor and adjust treatment plans.
Idiopathic Hypersomnia Treatment Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Idiopathic Hypersomnia Treatment, Gender-specific Diagnosed Incidence of Idiopathic Hypersomnia Treatment, Type-specific Diagnosed Incidence of Idiopathic Hypersomnia Treatment, Age-specific Diagnosed Incidence of Idiopathic Hypersomnia Treatment, Diagnosed Incident Population based on Primary Site of Idiopathic Hypersomnia Treatment, and Diagnosed Incident Population based on Histologic Classification of Idiopathic Hypersomnia Treatment Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of idiopathic hypersomnia treatment epidemiology in major markets worldwide.
Country Wise- Idiopathic Hypersomnia Treatment Multiforme Epidemiology
- The epidemiology segment provides Idiopathic Hypersomnia Treatment prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
-
Idiopathic Hypersomnia Treatment: Recent Developments:
- In August 2021, Jazz Pharmaceuticals announced that the FDA approved Xywav, a low-sodium oxybate oral solution, for idiopathic hypersomnia in adults. The approval marked the first FDAcleared treatment for this chronic sleep disorder. The drug received Fast Track, Priority Review, and Orphan Drug exclusivity, enabling expedited access and market protection.
-
Idiopathic Hypersomnia Treatment Marketed Drugs:
- Wakix: Harmony Biosciences
-
Wakix (pitolisant) is a histamine H3 receptor antagonist/inverse agonist that promotes wakefulness by enhancing histaminergic neurotransmission in the brain. Originally approved for narcolepsy, it received FDA approval in 2021 for treating idiopathic hypersomnia in adults. Wakix is notable for being a non-stimulant option and has a low risk of abuse or dependence.
- Xywav: Jazz Pharmaceuticals
-
Xywav (calcium, magnesium, potassium, and sodium oxybates) is a central nervous system depressant that modulates GABA activity to improve sleep quality and reduce excessive daytime sleepiness. FDA-approved in 2021 for idiopathic hypersomnia in adults, Xywav offers a lower-sodium alternative to Xyrem and is taken at night to help patients wake more refreshed.
- Provigil: Teva Pharmaceuticals
-
Provigil (modafinil) is a wakefulness-promoting agent used off-label for idiopathic hypersomnia. Though not FDA-approved specifically for IH, it is commonly prescribed due to its effectiveness in reducing excessive daytime sleepiness. Modafinil's exact mechanism is unclear, but it is believed to affect dopamine reuptake. It is considered a first-line treatment option.
• KP1077 (serdexmethylphenidate): an oral prodrug of methlphenidate, KP1077, showed clinically meaningful improvement in daytime sleepless, sleep inertia, and brain fog in phase 3=2 trails. It demonstrated good tolerability with both once daily evening and split dose regimens. A phase 3 study is expected to begin by he end of 2024
-
Pitolisant HD: An enhanced, higher dose vwesion of the wlak promoting H3 inverse against pitolisant (Wakix). Afteran fda refusal to file in early 2025, Harmony plans a pivotal phase 3 trail starting Q4 2025, aiming for approval by 2028oveporexton (TAK 861): A selective orexin 2 receptor against taken orally. As of Dec 2024, its in phase 3 trails for narcolepsy and IH, designed to directly enhanced wakefulness via the orexin system
danavorexton (TAK 925): An IV- administrated orexin 2 receptor against the mimics modafinil level wakefulness in phase 1 studies.
Idiopathic Hypersomnia Treatment Market Outlook
- The Idiopathic Hypersomnia Treatment Market refers to the pharmaceutical and therapeutic landscape aimed at managing excessive daytime sleepiness caused by idiopathic hypersomnia, a rare neurological disorder. It includes approved drugs, off-label treatments, emerging therapies, and supportive technologies that enhance patient alertness, cognition, and quality of life.
- Increasing awareness of sleep disorders, improved diagnostic tools, and growing demand for effective wake-promoting therapies are key market drivers. Rising incidence of hypersomnia, better healthcare access, and advancements in neuroscience and pharmacology are accelerating the development and adoption of novel treatment options for idiopathic hypersomnia.
- Opportunities lie in developing non-stimulant, low-abuse-potential therapies and orexin-based drugs. Expansion into underserved global markets, digital sleep diagnostics, and long-term management solutions also present growth potential. Strategic collaborations and personalized medicine are emerging as new frontiers in improving outcomes for idiopathic hypersomnia patients.
- Governments and health agencies are funding rare disease research, streamlining drug approval pathways, and promoting orphan drug development. Increased support from NIH, FDA fast-track programs, and patient advocacy efforts is encouraging innovation and accelerating access to idiopathic hypersomnia treatments worldwide.
- Limited disease awareness and underdiagnosis hinder early treatment and restrict market growth.
- The market is projected to grow significantly due to increasing R&D investment in orexin receptor agonists and next-generation wakefulness-promoting therapies.
Idiopathic Hypersomnia Treatment Market Segmentation
By Treatment Type:
- Pharmacological Treatments
- Stimulants
- Sodium Oxybate
- Antidepressants
- Modafinil and Armodafinil
- Non-Pharmacological Treatments
- Cognitive Behavioral Therapy (CBT)
- Sleep Hygiene Education
- Light Therapy
- Behavioural Interventions
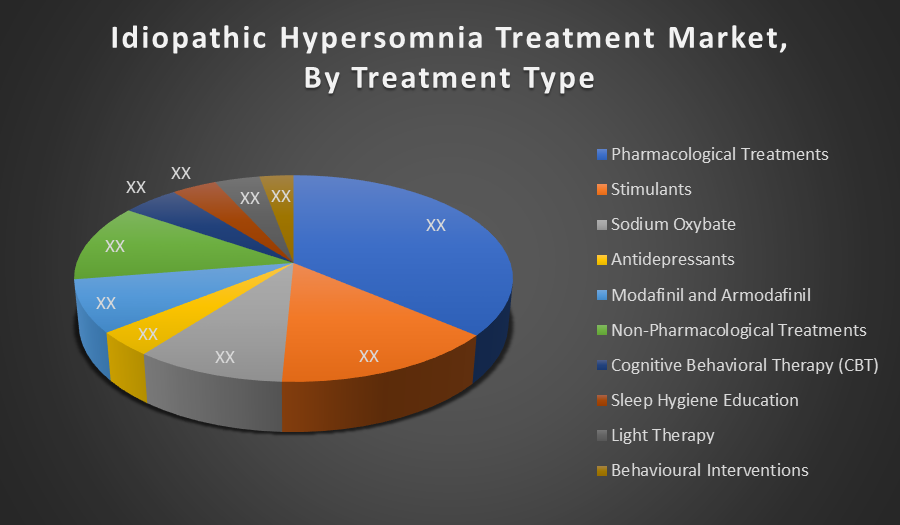
Pharmacological Treatments dominate the idiopathic hypersomnia treatment market due to their proven efficacy in controlling excessive daytime sleepiness. Drugs like modafinil, solriamfetol, and sodium oxybate are widely prescribed, supported by regulatory approvals and clinical guidelines. Their rapid symptom relief and widespread availability make them the preferred choice among patients and clinicians.
By Route of Administration:
- Oral
- Intravenous
- Transdermal
- Inhalatio
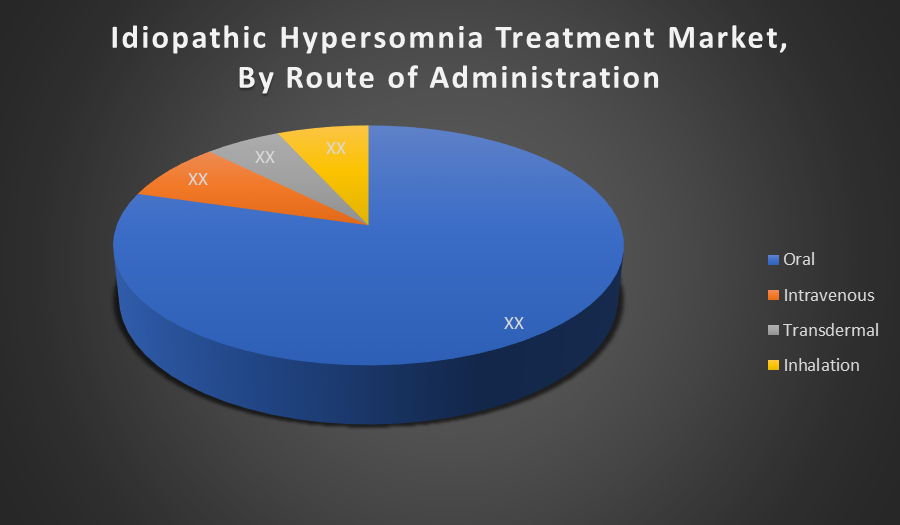
Oral administration holds the largest market share because it offers ease, convenience, and high patient compliance. Most approved therapies for idiopathic hypersomnia, including stimulants and oxybates, are formulated for oral use. The non-invasive nature of oral drugs and their suitability for long-term daily treatment further boost this segment’s dominance.
Regional Segment Analysis of the Idiopathic Hypersomnia Treatment Market
North America holds the largest share in the idiopathic hypersomnia treatment market due to its advanced healthcare infrastructure, widespread awareness of sleep disorders, and high diagnostic rates. The presence of key market players and early access to newly approved treatments like Xywav have strengthened the region’s dominance. Favourable reimbursement policies and active clinical research have also contributed to widespread patient access and treatment adherence across the United States and Canada.
Asia-Pacific is the fastest-growing region in the idiopathic hypersomnia treatment market, driven by rising healthcare investments, expanding urban populations, and growing awareness of sleep health. Improvements in diagnostic tools, increasing physician education, and broader availability of pharmacological treatments are accelerating market growth. Emerging economies are experiencing a surge in sleep clinic establishments and pharmaceutical imports, fostering early diagnosis and improved management of idiopathic hypersomnia across diverse patient populations.
Idiopathic Hypersomnia Treatment Market Key Companies
- Pfizer Inc.
- Harmony Biosciences
- Sun Pharmaceutical Industries
- Avadel Pharmaceuticals
- Eli Lilly and Company
- Jazz Pharmaceuticals
- Johnson & Johnson
- Teva Pharmaceuticals
- Bioprojet
- Merck & Co.
- Takeda Pharmaceutical Company
- Mylan N.V.
- Axsome Therapeutics
- Novartis AG
- NLS Pharmaceutics
- Others
Idiopathic Hypersomnia Therapeutics Market Report Scope
- The Idiopathic Hypersomnia therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Idiopathic Hypersomnia Treatment’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Idiopathic Hypersomnia Treatment therapies is provided, including an evaluation of new treatments expected to influence the current Idiopathic Hypersomnia Treatment market landscape.
- The report includes a detailed review of the Idiopathic Hypersomnia therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient-Based Idiopathic Hypersomnia Treatment Market Forecasting report offers valuable insights into trends shaping the global Idiopathic Hypersomnia Treatment market, helping to develop effective business strategies.
Idiopathic Hypersomnia Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Idiopathic Hypersomnia Therapeutic Approaches in Idiopathic Hypersomnia Treatment
- Review Of Drugs in Development for Idiopathic Hypersomnia Treatment
- Market, Growth, and Trends in Idiopathic Hypersomnia Treatment
- Market Opportunities in Idiopathic Hypersomnia Treatment
- Effects Of Future Therapies on Idiopathic Hypersomnia Treatment
Idiopathic Hypersomnia Treatment Market Report Key Strengths
- 15 Years Idiopathic Hypersomnia Treatment Market Forecast
- Global Coverage
- Idiopathic Hypersomnia Treatment Epidemiology Segmentation
- Key Cross Competition
Idiopathic Hypersomnia Treatment Market Report Assessment
- Present Practices in the Idiopathic Hypersomnia Treatment Market
- Review of Investigational Idiopathic Hypersomnia Treatment Drugs
- Attractiveness of the Idiopathic Hypersomnia Treatment Drug Market
- Idiopathic Hypersomnia Treatment Market Drivers
- Idiopathic Hypersomnia Treatment Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the idiopathic hypersomnia treatment market based on the below-mentioned segments:
Global Idiopathic Hypersomnia Treatment Market, By Treatment Type
- Pharmacological Treatments
- Stimulants
- Sodium Oxybate
- Antidepressants
- Modafinil and Armodafinil
- Non-Pharmacological Treatments
- Cognitive Behavioral Therapy (CBT)
- Sleep Hygiene Education
- Light Therapy
- Behavioural Interventions
Global Idiopathic Hypersomnia Treatment Market, By Route of Administration
- Oral
- Intravenous
- Transdermal
- Inhalation
Global Idiopathic Hypersomnia Treatment Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa