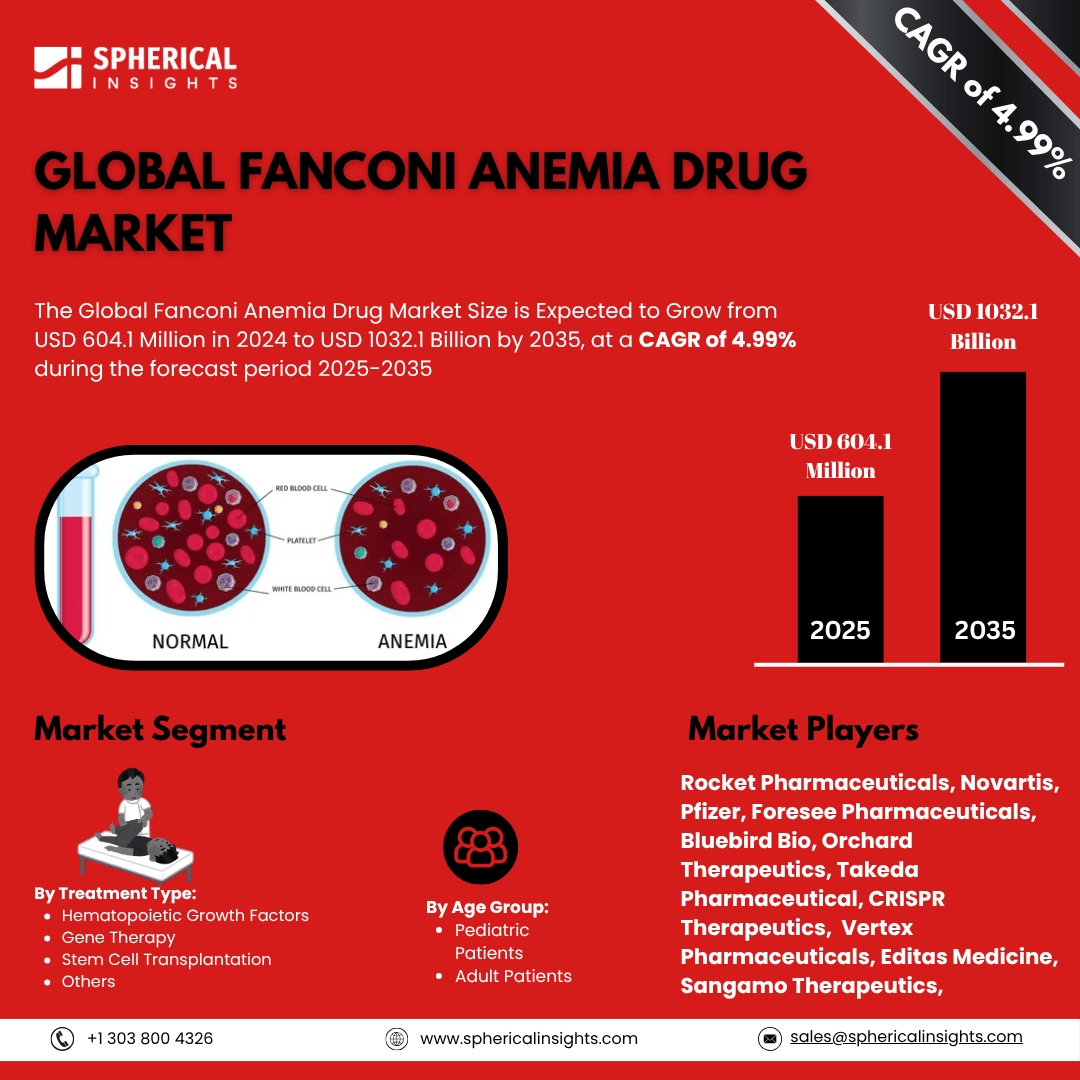
- As per Spherical Insights & Consulting, The Global Fanconi Anemia Drug Market Size is Expected to Grow from USD 604.1 Million in 2024 to USD 1032.1 Billion by 2035, at a CAGR of 4.99% during the forecast period 2025-2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Fanconi Anemia Drug Market Companies such as Rocket Pharmaceuticals, Novartis, Pfizer, Foresee Pharmaceuticals, Bluebird Bio, Orchard Therapeutics, Takeda Pharmaceutical, CRISPR Therapeutics, Vertex Pharmaceuticals, Editas Medicine, Sangamo Therapeutics, GlaxoSmithKline (GSK), AstraZeneca, Bayer AG, BioMarin Pharmaceutical, and Others.
Fanconi Anemia Drug Treatment Market: Understanding and Treatment Algorithm:
Fanconi Anemia is a rare inherited genetic disorder that affects the bone marrow, leading to decreased production of blood cells. It causes physical abnormalities, increased risk of leukemia, and other cancers. The condition is typically diagnosed in childhood and results from mutations in genes responsible for DNA repair and cell stability.
Fanconi Anemia Drug Diagnosis:
Diagnosis of Fanconi Anemia involves a combination of clinical evaluation, family history, and specialized laboratory tests. The most definitive test is the chromosomal breakage test using diepoxybutane (DEB) or mitomycin C, which identifies DNA repair defects. Genetic testing can confirm mutations in FA-related genes, aiding in early and accurate diagnosis.
Fanconi Anemia Drug Treatment:
Treatment for Fanconi Anemia focuses on managing symptoms and preventing complications. Common approaches include blood transfusions, hematopoietic growth factors, and androgens to support blood cell production. The only curative option is stem cell transplantation. Gene therapy and emerging targeted treatments are being explored to correct underlying genetic defects and improve long-term outcomes.
Fanconi Anemia Drug Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Fanconi Anemia Drug, Gender-specific Diagnosed Incidence of Fanconi Anemia Drug, Type-specific Diagnosed Incidence of Fanconi Anemia Drug, Age-specific Diagnosed Incidence of Fanconi Anemia Drug, Diagnosed Incident Population based on Primary Site of Fanconi Anemia Drug, and Diagnosed Incident Population based on Histologic Classification of Fanconi Anemia Drug Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of Fanconi anemia drug epidemiology in major markets worldwide.
Country-Wise Fanconi Anemia Drug Multiforme Epidemiology
- The epidemiology segment provides Fanconi Anemia Drug prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
Fanconi Anemia Drug Recent Developments:
- In April 2024, Rocket Pharmaceuticals announced that the European Medicines Agency (EMA) accepted its Marketing Authorization Application for RP L102, an investigational gene therapy for Fanconi Anemia. The application was based on positive clinical data demonstrating sustained genetic correction and hematologic improvement. The therapy was well tolerated without the need for cytotoxic conditioning.
Fanconi Anemia Drug Marketed Drugs:
- Oxymetholone: Pfizer Inc.
Oxymetholone is an anabolic androgenic steroid used to stimulate red blood cell production in Fanconi Anemia patients with anemia. It helps improve haemoglobin levels and delays the need for blood transfusions or bone marrow transplant. Though not curative, it is commonly prescribed for temporary hematologic support in pediatric and adult FA patients.
Eltrombopag is a thrombopoietin receptor agonist that stimulates platelet production. While not FA-specific, it is sometimes used off-label in Fanconi Anemia patients with severe thrombocytopenia to reduce bleeding risks. It may serve as a supportive treatment before or after stem cell transplantation.
- RP-L102: Rocket Pharmaceuticals
RP-L102 is an investigational gene therapy for FA patients with FANCA mutations. It uses autologous CD34 stem cells modified with a lentiviral vector to restore DNA repair function. It has received FDA Orphan Drug and RMAT designations and is in advanced clinical development, aiming to provide a curative option without chemotherapy.
Fanconi Anemia Drug: Emerging Therapies
- FP-045; It is an oral small molecules in phase I/II trails, enhancing the ALDH2 enzyme to mitigate aldehyde included oxidative stress in FA stem cells. By improving Mitochondrial detoxification, its aims to preserve bone function. It represents a novel non-gene therapy approach targeting the underlying cellular pathology