Asia Pacific Drug Reference Apps Market Size, Share, and COVID-19 Impact Analysis, By Device (Smartphones and Tablets), By End Use (Healthcare Professionals, Patients, Pharmacists, Researchers and Educators, and Others), and Asia Pacific Drug Reference Apps Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareAsia Pacific Drugs Referance Apps Market Insights Forecasts to 2035
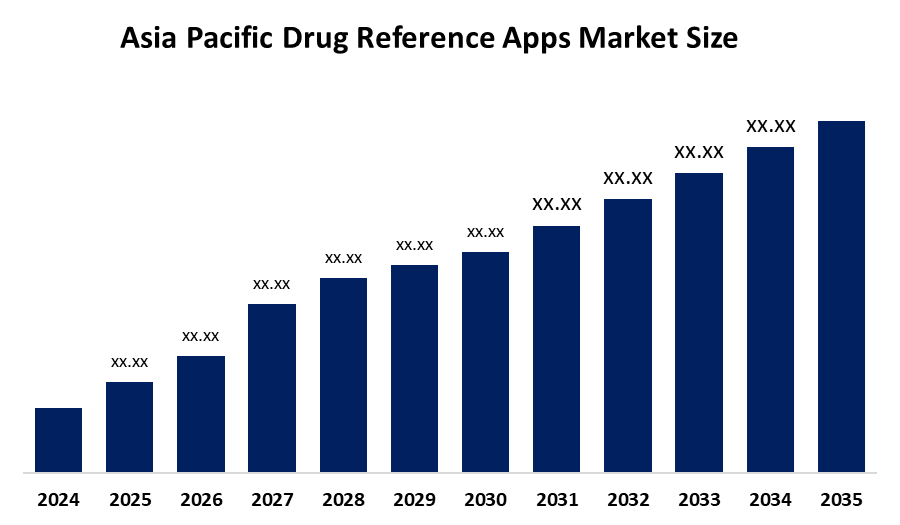
•Asia Pacific Drugs Referance Apps Market Size is expected to grow at a CAGR of around 7.9% from 2025 to 2035
•Asia Pacific Drugs Referance Apps Market Size is expected to hold a significant share by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, The Asia Pacific Drug Reference apps Market Size is Growing at a CAGR of 7.9% from 2025 to 2035. The drug reference apps market in Asia Pacific is driven by high smartphone penetration, increasing internet access, rising chronic diseases, and a greater focus on digital health solutions, with strong potential in China, India, and Japan
.
Market Overview
The Asia Pacific drug reference apps market refers to the industry of mobile and web-based applications designed to provide healthcare professionals and patients with immediate access to comprehensive drug databases, including information on dosages, side effects, and contraindications. This market is characterized by a high demand for real-time, evidence-based data at the point of care to reduce prescription errors and improve clinical outcomes. Key trends include the integration of these apps with electronic health records (EHRs) and a shift toward personalized medicine, where apps analyse patient-specific data to provide tailored drug recommendations.
Governments across Asia Pacific are actively promoting digital health through initiatives such as "Healthy China 2030" and India’s expanding mobile health ecosystem, which foster the adoption of medical apps. Private sector efforts are focused on strategic partnerships between app developers and pharmaceutical companies to ensure that drug information remains current and medically verified. These collaborative efforts aim to harmonize data standards and strengthen regulatory frameworks to scale digital health solutions responsibly across the region.
Technological advancements are revolutionizing the market through the integration of Artificial Intelligence (AI) and machine learning. AI-powered features now allow for advanced drug-drug interaction checks and the prediction of adverse reactions by analyzing comorbidities and concurrent medications. Additionally, advancements in mobile connectivity and cloud-based delivery systems have expanded the accessibility of these apps, enabling their use in virtual consultations and remote patient monitoring in previously underserved rural areas.
Report Coverage
This research report categorizes the market for the Asia Pacific drug reference apps market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Asia Pacific drug reference apps market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Asia Pacific drug reference apps market.
Asia Pacific Drug Reference Apps Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR of 7.9% |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 210 |
| Tables, Charts & Figures: | 90 |
| Segments covered: | By Device |
| Companies covered:: | AstraZeneca Pharma India, Kowa Company, Ltd., Astellas Pharma Inc., Sanwa Kagaku Kenkyusho Co., Ltd., Steadfast MediShield Pvt. Ltd., RSM Kilitch Pharma Pvt. Ltd., Klarvoyant Biogenics Pvt. Ltd., Takeda Pharmaceutical Company Limited, Dr. Reddy’s Laboratories, Sun Pharmaceutical Industries Ltd., and and Other key players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The market is primarily driven by the increasing adoption of mobile health technologies and the rising need for accurate drug information among healthcare professionals to ensure patient safety. The global rise in chronic diseases and an aging population in countries like China and Japan have heightened the demand for reliable drug management tools. Furthermore, the expansion of telemedicine and remote healthcare has made mobile drug reference apps indispensable for safe prescribing during virtual consultations, particularly in rural regions.
Restraining Factors
Market growth is hindered by concerns over data privacy and the accuracy of information provided by unverified apps. High development costs and stringent regulatory requirements across different jurisdictions pose significant hurdles for developers. Additionally, the lack of universal healthcare coverage in some Southeast Asian countries restricts market penetration.
Market Segmentation
The Asia Pacific drug reference apps market share is classified into device and end use.
- The smartphones segment dominated the market in 2024 and is expected to grow at a remarkable CAGR during the forecast period.
The Asia Pacific drug reference apps market is segmented by device into smartphones and tablets. Among these, the smartphones segment dominated the market in 2024 and is expected to grow at a remarkable CAGR during the forecast period. This is due to advanced features like AI-driven clinical decision support and real-time drug tracking are rapidly being incorporated into smartphone-based (DRA) systems to improve patient safety and healthcare workers' productivity.
- The healthcare professionals segment dominated the market in 2024 and is anticipated to grow at a substantial CAGR during the forecast period.
The Asia Pacific drug reference apps market is segmented by end use into healthcare professionals, patients, pharmacists, researchers and educators, and others. Among these, the healthcare professionals segment dominated the market in 2024 and is anticipated to grow at a substantial CAGR during the forecast period. In order to improve patient safety and optimize clinical processes, healthcare professionals are rapidly implementing advanced clinical decision support (DRA) systems that incorporate AI-driven clinical decision support, real-time medication tracking, and tailored therapy recommendations.
Competitive Analysis
The report offers the appropriate analysis of the key organizations/companies involved within the Asia Pacific drug reference apps market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- MIMS
- Haleon PLC
- Dr. Reddy’s Laboratories
- Samsung Biologics
- Eisai Co., Ltd.
- Kyowa Kirin
- Takeda Pharmaceutical Company
- Cipla
- Sun Pharmaceutical Industries
- Aurobindo Pharma
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the Asia Pacific, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Asia Pacific drug reference apps market based on the below-mentioned segments:
Asia Pacific Drug Reference Apps Market, By Device
- Smartphones
- Tablets
Asia Pacific Drug Reference Apps Market, By End Use
- Healthcare Professionals
- Patients
- Pharmacists
- Researchers and Educators
- Others
Frequently Asked Questions (FAQ)
-
What is the current growth outlook for the Asia Pacific drug reference apps market?The market is expected to grow at a CAGR of 7.9% from 2025 to 2035, driven by rising smartphone adoption among healthcare professionals and increasing demand for real-time drug information.
-
Which device segment dominates the market?The smartphones segment dominated in 2024 due to portability, real-time drug tracking, and integration with AI-driven clinical decision support systems.
Need help to buy this report?