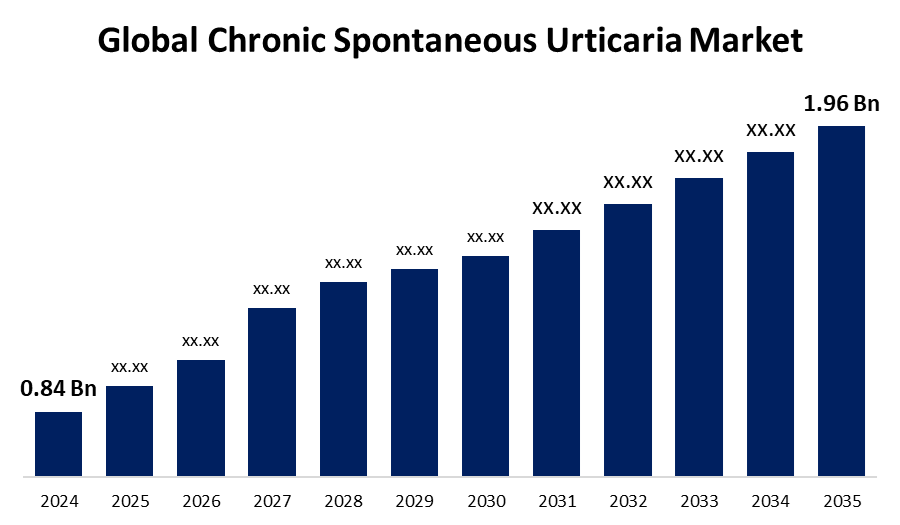
- As per Spherical Insights & Consulting, The Global Chronic Spontaneous Urticaria Market Size is Expected to Grow from USD 0.84 Billion in 2024 to USD 1.96 Billion by 2035, at a CAGR of 8.84% during the forecast period 2025-2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Chronic Spontaneous Urticaria Market Companies such as Roche, Bristol Myers Squibb, Merck & Co., AstraZeneca, Pfizer, Novartis, Johnson & Johnson, Amgen, Eli Lilly, Sanofi, Takeda, Bayer, GlaxoSmithKline, AbbVie, Genentech, and Others.
Chronic Spontaneous Urticaria Treatment Market: Understanding and Treatment Algorithm:
Chronic Spontaneous Urticaria (CSU) is a persistent skin condition marked by spontaneous, recurring hives and intense itching lasting over six weeks. It significantly impacts quality of life, with complex causes involving immune system dysregulation. It requires tailored diagnosis and treatment for effective symptom control.
Chronic Spontaneous Urticaria Diagnosis:
Diagnosis primarily involves a thorough physical examination and detailed patient history to identify characteristic hives lasting more than six weeks. Additional tests, such as blood tests, allergy tests, and autoimmune screenings, help rule out underlying causes, ensuring an accurate diagnosis and personalized treatment plans.
Chronic Spontaneous Urticaria Treatment
Treatment focuses on symptom relief using antihistamines as first-line therapy. For resistant cases, biologics like omalizumab, corticosteroids, and immunosuppressants are used. Phototherapy and lifestyle changes may also support management, aiming to improve quality of life and reduce flare-ups.
Chronic Spontaneous Urticaria Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Chronic Spontaneous Urticaria, Gender-specific Diagnosed Incidence of Chronic Spontaneous Urticaria, Type-specific Diagnosed Incidence of Chronic Spontaneous Urticaria, Age-specific Diagnosed Incidence of Chronic Spontaneous Urticaria, Diagnosed Incident Population based on Primary Site of Chronic Spontaneous Urticaria, and Diagnosed Incident Population based on Histologic Classification of Chronic Spontaneous Urticaria Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of Chronic Spontaneous Urticaria epidemiology in major markets worldwide.
Country-Wise- Chronic Spontaneous Urticaria Multiforme Epidemiology
- The epidemiology segment provides Chronic Spontaneous Urticaria prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
Chronic Spontaneous Urticaria: Recent Developments:
- In April 2025, Sanofi and Regeneron secured FDA approval for Dupixent in April 2025 to treat chronic spontaneous urticaria (CSU) in patients aged 12 and older. This followed a prior 2023 rejection due to limited efficacy data. The expanded indication now addresses over 300,000 U.S. patients.
Chronic Spontaneous Urticaria Marketed Drugs:
- Dupixent: Sanofi & Regeneron
Dupixent (dupilumab) is a monoclonal antibody that inhibits interleukin-4 and interleukin-13 signaling, key drivers of type 2 inflammation. The FDA approved its use in adolescents and adults (12+ years) with CSU who remain symptomatic despite antihistamine treatment. This expanded its indications beyond asthma and atopic dermatitis.
- Xolair: Genentech & Novartis
Xolair (omalizumab) is an anti-IgE monoclonal antibody. It binds to circulating IgE, preventing activation of mast cells and basophils. FDA-approved for moderate-to-severe persistent asthma and chronic idiopathic urticaria (a form of CSU), it is used when antihistamines fail to provide relief.
- Nucala: GlaxoSmithKline (GSK)
Nucala (mepolizumab) is an anti-IL-5 monoclonal antibody that reduces eosinophilic inflammation. Although primarily approved for severe eosinophilic asthma and eosinophilic granulomatosis with polyangiitis (EGPA), it has shown off-label potential in urticaria driven by eosinophil activity.
Chronic Spontaneous Urticaria: Emerging Therapies
- Ligelizumab: A next-generation humanized monoclonal antibody that binds free IgE with greater affinity than omalizumab. It neutralizes IgE more effectively, leading to better control of hives and itching. Clinical trials have shown superior symptom relief and longer duration of action.
- AK002 (Lirentelimab): This is an anti-Siglec-8 monoclonal antibody that targets and depletes eosinophils and inhibits mast cell activation. Reducing these inflammatory cells helps decrease allergic inflammation and symptoms in patients with refractory CSU.
- BNT327: A bispecific antibody currently in late-stage clinical trials. It targets two immune checkpoints to boost T-cell activation and improve immune regulation, aiming to help patients who do not respond to standard PD-1/PD-L1 therapies.
- Dupilumab: An IL-4 and IL-13 receptor antagonist approved for atopic dermatitis and asthma, now being studied off-label for CSU. By blocking these cytokines, it reduces inflammation and immune overactivity associated with chronic urticaria.
Chronic Spontaneous Urticaria Market Outlook
- The Global Chronic Spontaneous Urticaria Market involves the diagnosis, treatment, and management of a persistent skin condition characterized by spontaneous hives and itching. It includes medications, therapies, and diagnostic tools aimed at improving patient quality of life and controlling symptoms effectively worldwide.
- Key drivers of the Chronic Spontaneous Urticaria market include rising disease prevalence, increasing awareness, advancements in biologic therapies, growing healthcare expenditure, and improved diagnostic techniques. Additionally, expanding patient access to treatment and supportive government initiatives fuel market growth globally.
- Market opportunities include expanding biologic treatment adoption, untapped emerging markets, growing patient awareness, advancements in personalized medicine, and development of novel therapies. Increasing healthcare infrastructure and rising investments in research also present significant growth potential in the Chronic Spontaneous Urticaria market.
- Government Programs like Ayushman Bharat and National Health Mission enhance healthcare infrastructure and provide subsidized medicines, boosting patient outcomes globally.
- Limited awareness and high treatment costs hinder widespread diagnosis and effective management of Chronic Spontaneous Urticaria globally.
- The Chronic Spontaneous Urticaria market is projected to grow steadily, driven by rising prevalence and advances in targeted therapies.
Chronic Spontaneous Urticaria Market Segmentation
By Treatment:
- Medication
- Phototherapy
- Others
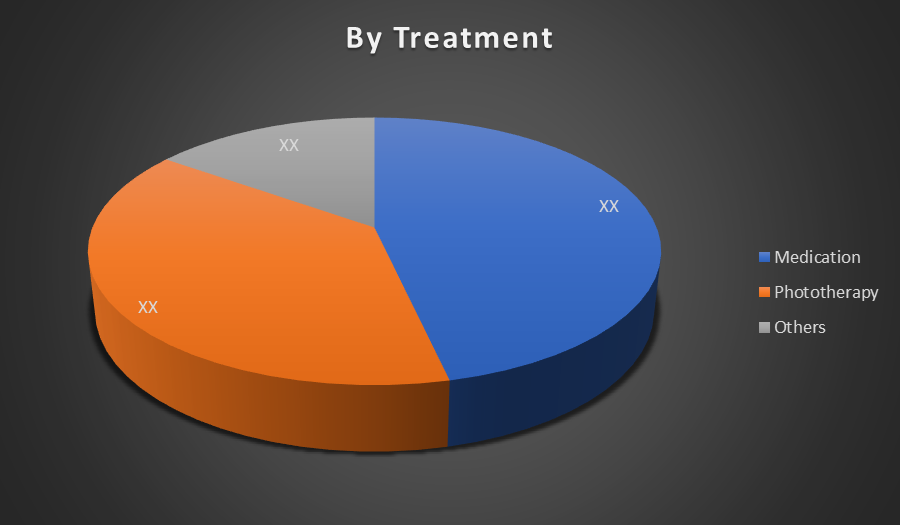
The Medication segment dominates the Global Chronic Spontaneous Urticaria market due to its effectiveness in symptom relief, widespread availability, and physician preference. Antihistamines and biologics like omalizumab offer targeted treatment, rapid results, and manageable side effects, making them the most relied-upon therapy by patients and clinicians.
By Diagnosis:
- Physical Examination
- Blood Test
- Allergy Test
- Others
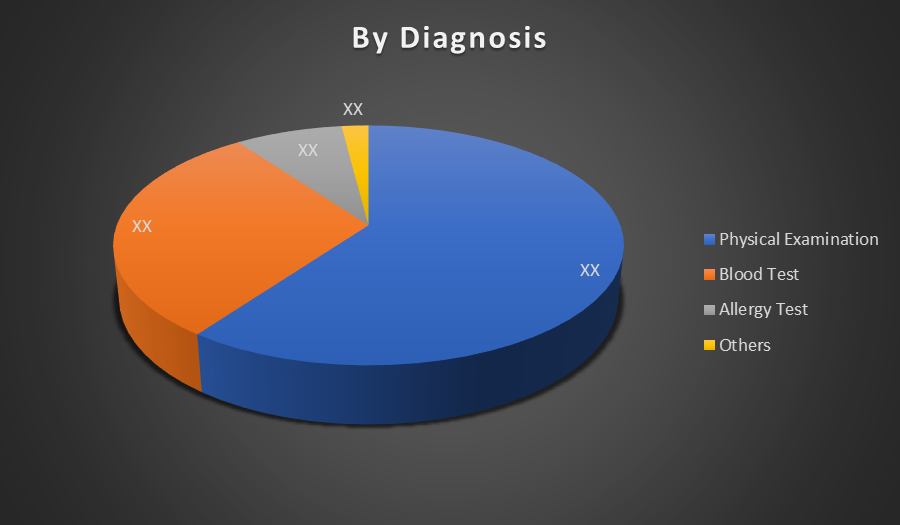
The Physical Examination segment dominates the Chronic Spontaneous Urticaria market by diagnosis due to its simplicity, cost-effectiveness, and immediate accessibility. Physicians often rely on visible symptoms and patient history to diagnose, making it the first-line and most commonly used diagnostic method in clinical practice.
Regional Segment Analysis of the Chronic Spontaneous Urticaria Market
North America dominates the Chronic Spontaneous Urticaria market due to its advanced healthcare infrastructure, high awareness levels, and widespread access to novel therapies like biologics. The region also benefits from strong research initiatives, favorable reimbursement policies, and a high prevalence of allergic disorders, driving demand for effective treatment and early diagnosis across the population.
Asia Pacific is the fastest-growing region in the Chronic Spontaneous Urticaria market due to its large population base, growing healthcare spending, and increasing disease awareness. Enhanced medical infrastructure, rising access to advanced therapies, and supportive government policies in countries like China, India, and Japan are driving rapid market expansion.
Chronic Spontaneous Urticaria Market Key Companies
- Roche
- Bristol Myers Squibb
- Merck & Co.
- AstraZeneca
- Pfizer
- Novartis
- Johnson & Johnson
- Amgen
- Eli Lilly
- Sanofi
- Takeda
- Bayer
- GlaxoSmithKline
- AbbVie
- Genentech
- Others
Chronic Spontaneous Urticaria Therapeutics Market Report Scope
- The Chronic Spontaneous Urticaria therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Chronic Spontaneous Urticaria’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Chronic Spontaneous Urticaria therapies is provided, including an evaluation of new treatments expected to influence the current Chronic Spontaneous Urticaria treatment market landscape.
- The report includes a detailed review of the Chronic Spontaneous Urticaria therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient-Based Chronic Spontaneous Urticaria Market Forecasting report offers valuable insights into trends shaping the global Chronic Spontaneous Urticaria market, helping to develop effective business strategies.
Chronic Spontaneous Urticaria Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Chronic Spontaneous Urticaria Therapeutic Approaches in Chronic Spontaneous Urticaria
- Review Of Drugs in Development for Chronic Spontaneous Urticaria
- Market, Growth, and Trends in Chronic Spontaneous Urticaria
- Market Opportunities in Chronic Spontaneous Urticaria Treatment
- Effects Of Future Therapies on Chronic Spontaneous Urticaria Treatment.
Chronic Spontaneous Urticaria Treatment Market Report Key Strengths
- 15 Years Chronic Spontaneous Urticaria Market Forecast
- Global Coverage
- Chronic Spontaneous Urticaria Epidemiology Segmentation
- Key Cross Competition
Chronic Spontaneous Urticaria Treatment Market Report Assessment
- Present Practices in the Chronic Spontaneous Urticaria Treatment Market
- Review of Investigational Chronic Spontaneous Urticaria Drugs
- Attractiveness of the Chronic Spontaneous Urticaria Drug Market
- Chronic Spontaneous Urticaria Market Drivers
- Chronic Spontaneous Urticaria Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the chronic spontaneous urticaria market based on the below-mentioned segments:
Global Chronic Spontaneous Urticaria Market, By Treatment
- Medication
- Phototherapy
- Others
Global Chronic Spontaneous Urticaria Market, By Diagnosis
- Physical Examination
- Blood Test
- Allergy Test
- Others
Global Chronic Spontaneous Urticaria Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa






