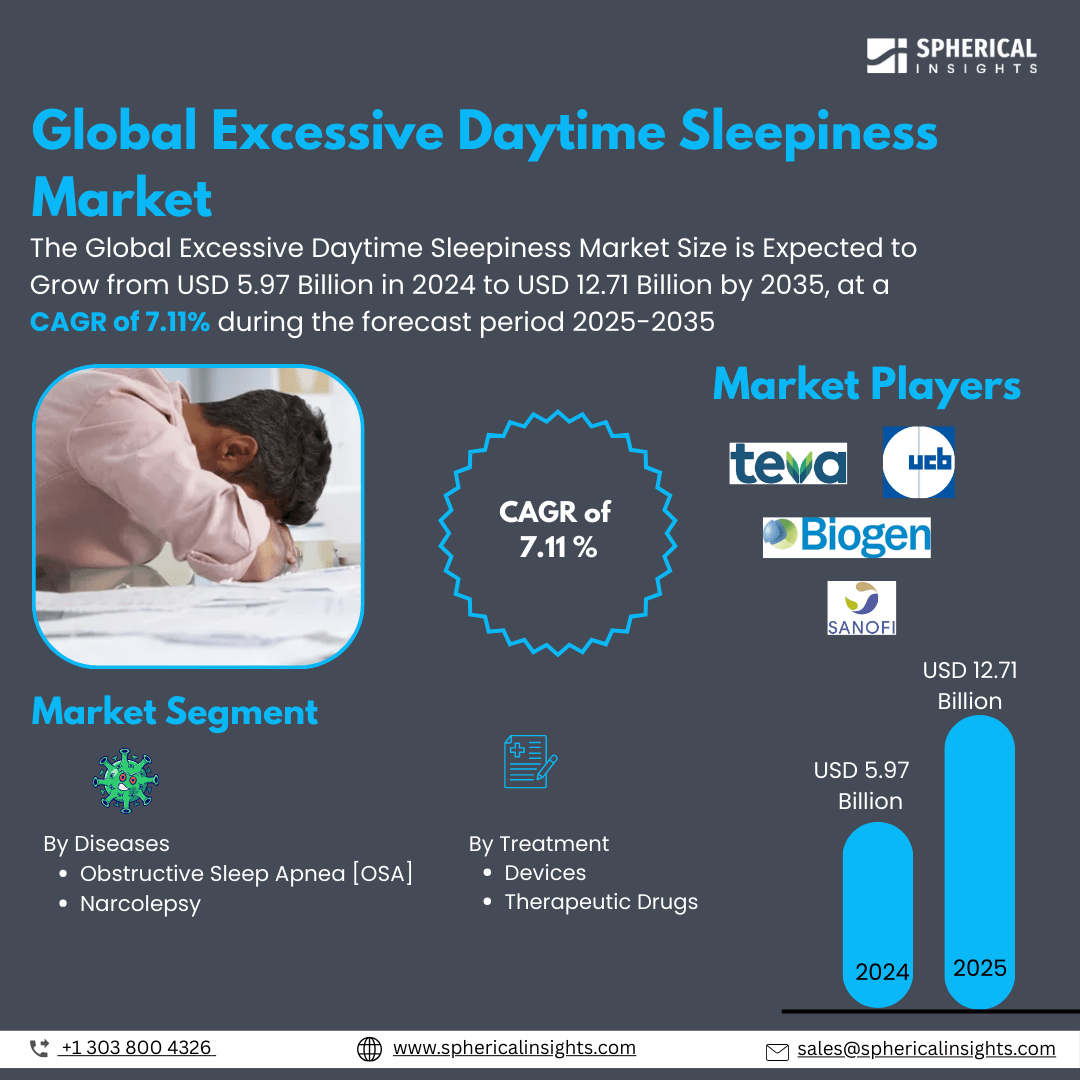
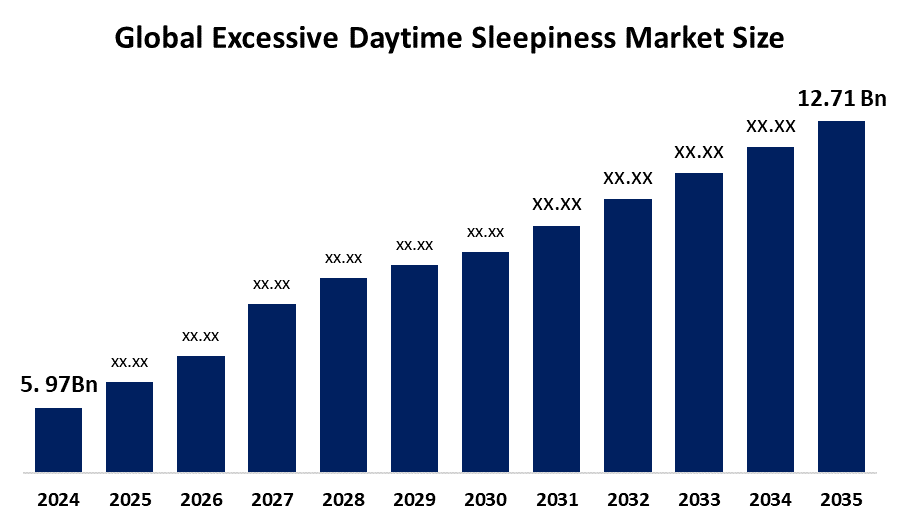
- As per Spherical Insights & Consulting, The Global Excessive Daytime Sleepiness Market Size is Expected To Grow from USD 5.97 Billion in 2024 to USD 12.71 Billion by 2035, at a CAGR of 7.11% during the forecast period 2025-2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Excessive Daytime Sleepiness Market Companies such as Teva Pharmaceuticals, Jazz Pharmaceuticals, Harmony Biosciences, Eisai Co., Biogen, UCB Pharma, Biogen Idec, Pfizer Inc., Merck & Co., Sanofi, Novartis, Takeda Pharmaceutical, Eli Lilly and Company, Sunovion Pharmaceuticals, Lundbeck, and Others.
Excessive Daytime Sleepiness Treatment Market: Understanding and Treatment Algorithm:
Excessive Daytime Sleepiness (EDS) Market Size is a condition characterized by persistent and overwhelming drowsiness during the day, even after adequate nighttime sleep. It often impairs daily functioning, attention, and alertness, and is commonly associated with sleep disorders like narcolepsy and obstructive sleep apnea.
Excessive Daytime Sleepiness Diagnosis:
Diagnosis of Excessive Daytime Sleepiness (EDS) involves a combination of clinical evaluation, patient history, and specialized tests. Common methods include the Epworth Sleepiness Scale (ESS) to assess sleepiness severity, polysomnography (sleep study) to monitor sleep patterns, and the Multiple Sleep Latency Test (MSLT) to measure daytime sleepiness and diagnose underlying sleep disorders.
Excessive Daytime Sleepiness Treatment
Treatment focuses on addressing the underlying cause and managing symptoms. It includes lifestyle changes like improved sleep hygiene, use of devices such as CPAP for sleep apnea, and medications like stimulants or wakefulness-promoting agents (e.g., modafinil). Behavioural therapies may also support better daytime alertness.
Excessive Daytime Sleepiness Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Excessive Daytime Sleepiness, Gender-specific Diagnosed Incidence of Excessive Daytime Sleepiness, Type-specific Diagnosed Incidence of Excessive Daytime Sleepiness, Age-specific Diagnosed Incidence of Excessive Daytime Sleepiness, Diagnosed Incident Population based on Primary Site of Excessive Daytime Sleepiness, and Diagnosed Incident Population based on Histologic Classification of Excessive Daytime Sleepiness Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of Excessive Daytime Sleepiness epidemiology in major markets worldwide.
Country Wise- Excessive Daytime Sleepiness Multiforme Epidemiology
- The epidemiology segment provides Excessive Daytime Sleepiness prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
Excessive Daytime Sleepiness: Recent Developments:
- In October 2024, Avadel Pharmaceuticals announced that the U.S. FDA had expanded the approval of LUMRYZ™ (sodium oxybate) to include pediatric patients aged 7 and older with narcolepsy. This extended approval allows LUMRYZ to treat both cataplexy and excessive daytime sleepiness in children, providing a once-at-bedtime dosing option. The approval was supported by positive data from the REST-ON Phase 3 trial, which demonstrated significant improvements in key symptoms of narcolepsy.
Excessive Daytime Sleepiness Marketed Drugs:
- Nuvigil: Cephalon (Teva Pharmaceuticals)
Nuvigil (armodafinil) is a wakefulness-promoting agent approved to treat excessive daytime sleepiness caused by narcolepsy, obstructive sleep apnea, and shift work sleep disorder. It works by altering brain neurotransmitters to enhance alertness and reduce fatigue.
- Provigil: Cephalon (Teva Pharmaceuticals)
Provigil (modafinil) is a stimulant approved for excessive daytime sleepiness linked to narcolepsy, obstructive sleep apnea, and shift work sleep disorder. It promotes wakefulness by modulating neurotransmitter pathways in the brain.
- Xyrem: Jazz Pharmaceuticals
Xyrem (sodium oxybate) is FDA-approved for narcolepsy-related excessive daytime sleepiness and cataplexy. It improves nighttime sleep quality, reducing daytime symptoms by acting as a central nervous system depressant.
Excessive Daytime Sleepiness: Emerging Therapies
- BNT327: It is a bispecific antibody in late-stage trials for Excessive Daytime Sleepiness. It targets two immune checkpoints to enhance T-cell activation, aiming to improve treatment outcomes in patients with sleep disorders resistant to current therapies.
- Solriamfetol: It is a dopamine and norepinephrine reuptake inhibitor approved recently for treating excessive daytime sleepiness in patients with narcolepsy and obstructive sleep apnea. It improves wakefulness by enhancing neurotransmitter activity, offering a non-traditional option with fewer side effects than stimulants.
- Pitolisant: It is an H3 receptor antagonist/inverse agonist in clinical development for Excessive Daytime Sleepiness. It promotes histamine release in the brain, improving wakefulness and reducing sleepiness without the addictive risks associated with traditional stimulants.
- TAK-925: It is a selective orexin 2 receptor agonist under investigation for narcolepsy-related daytime sleepiness. It targets the orexin system to regulate sleep-wake cycles, showing promise in improving wakefulness and reducing symptoms in patients with sleep disorders.
Excessive Daytime Sleepiness Market Outlook
- The Excessive Daytime Sleepiness market involves the diagnosis and treatment of conditions causing abnormal daytime drowsiness, primarily due to disorders like obstructive sleep apnea and narcolepsy. It includes devices and therapeutic drugs designed to improve alertness and quality of life for affected individuals.
- Key drivers include increasing prevalence of sleep disorders, growing awareness about their health impact, rising obesity rates, and advancements in diagnostic and treatment technologies. Additionally, the aging global population and expanding healthcare infrastructure further fuel market growth.
- Opportunities lie in emerging markets with rising healthcare access, development of innovative non-invasive devices, personalized therapies, and integration of digital health solutions. Expanding insurance coverage and increasing research investments also provide avenues for market expansion.
- Governments worldwide support sleep health by funding research, raising awareness campaigns, and implementing policies for better diagnosis and treatment access. These initiatives aim to reduce the burden of sleep-related disorders on public health systems.
- Patient non-compliance with device usage and limited awareness in certain regions hinder market growth.
- The market is projected to grow steadily due to the increasing prevalence of sleep disorders and technological advancements improving diagnosis and treatment effectiveness.
Excessive Daytime Sleepiness Market Segmentation
By Diseases:
- Obstructive Sleep Apnea [OSA]
- Narcolepsy
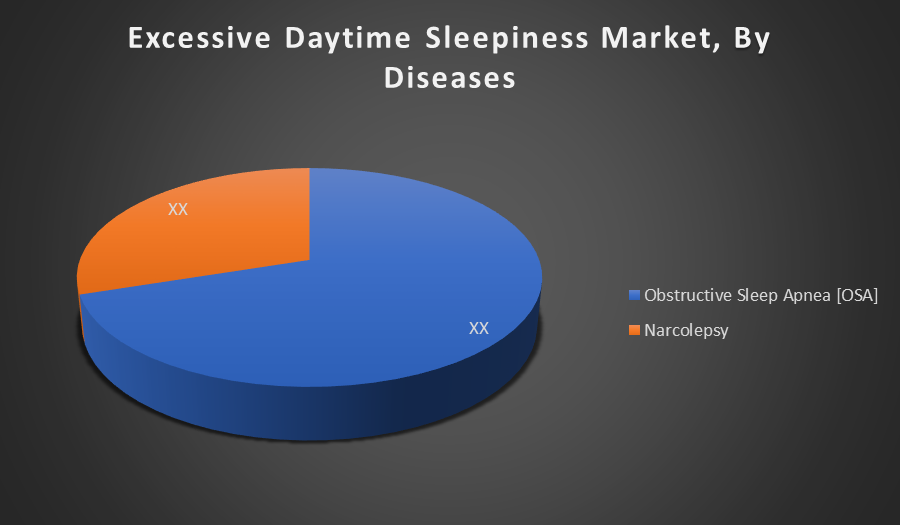
Obstructive Sleep Apnea (OSA) holds the largest share in the Excessive Daytime Sleepiness market due to its higher prevalence globally compared to narcolepsy. OSA is more commonly diagnosed, driven by increasing obesity rates and aging populations, which contribute to airway obstruction during sleep and subsequent daytime sleepiness.
By Treatment:
- Devices
- Therapeutic Drugs
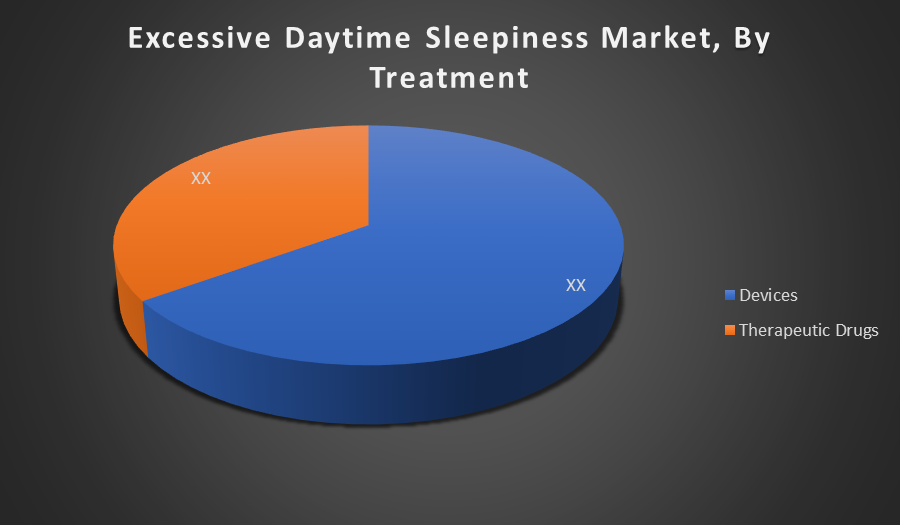
Devices dominate the market share because continuous positive airway pressure (CPAP) machines are the standard and most effective treatment for OSA-related sleepiness. Their widespread adoption and insurance coverage, along with advances in device technology, drive their preference over therapeutic drugs, which often have limited efficacy and more side effects.
Regional Segment Analysis of the Excessive Daytime Sleepiness Market
North America holds the largest share in the Excessive Daytime Sleepiness market, driven by high awareness, advanced healthcare infrastructure, and widespread adoption of diagnostic and treatment devices. The region benefits from extensive research funding, insurance coverage, and a large population affected by sleep disorders like obstructive sleep apnea, fueling demand for effective therapies and devices that improve patient outcomes and quality of life.
The Asia-Pacific region is the fastest-growing market for Excessive Daytime Sleepiness due to rising awareness of sleep disorders, increasing healthcare investments, and a growing diabetic and obese population. Rapid urbanization, improving diagnostic facilities, and expanding healthcare access contribute to the increasing adoption of sleep apnea devices and medications, making this region a key area for market expansion and innovation in treatment solutions.
Excessive Daytime Sleepiness Market Key Companies
- Teva Pharmaceuticals
- Jazz Pharmaceuticals
- Harmony Biosciences
- Eisai Co.
- Biogen
- UCB Pharma
- Biogen Idec
- Pfizer Inc.
- Merck & Co.
- Sanofi
- Novartis
- Takeda Pharmaceutical
- Eli Lilly and Company
- Sunovion Pharmaceuticals
- Lundbeck
- Others
Excessive Daytime Sleepiness Therapeutics Market Report Scope
- The Excessive Daytime Sleepiness therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Excessive Daytime Sleepiness’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Excessive Daytime Sleepiness therapies is provided, including an evaluation of new treatments expected to influence the current Excessive Daytime Sleepiness treatment market landscape.
- The report includes a detailed review of the Excessive Daytime Sleepiness therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient-Based Excessive Daytime Sleepiness Market Forecasting report offers valuable insights into trends shaping the global Excessive Daytime Sleepiness market, helping to develop effective business strategies.
Excessive Daytime Sleepiness Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Excessive Daytime Sleepiness Therapeutic Approaches in Excessive Daytime Sleepiness
- Review Of Drugs in Development for Excessive Daytime Sleepiness
- Market, Growth, and Trends in Excessive Daytime Sleepiness
- Market Opportunities in Excessive Daytime Sleepiness Treatment
- Effects Of Future Therapies on Excessive Daytime Sleepiness Treatment.
Excessive Daytime Sleepiness Treatment Market Report Key Strengths
- 15 Years Excessive Daytime Sleepiness Market Forecast
- Global Coverage
- Excessive Daytime Sleepiness Epidemiology Segmentation
- Key Cross Competition
Excessive Daytime Sleepiness Treatment Market Report Assessment
- Present Practices in the Excessive Daytime Sleepiness Treatment Market
- Review of Investigational Excessive Daytime Sleepiness Drugs
- Attractiveness of the Excessive Daytime Sleepiness Drug Market
- Excessive Daytime Sleepiness Market Drivers
- Excessive Daytime Sleepiness Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the excessive daytime sleepiness market based on the below-mentioned segments:
Global Excessive Daytime Sleepiness Market, By Diseases
- Obstructive Sleep Apnea [OSA]
- Narcolepsy
Global Excessive Daytime Sleepiness Market, By Treatment
- Devices
- Therapeutic Drugs
Global Excessive Daytime Sleepiness Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa