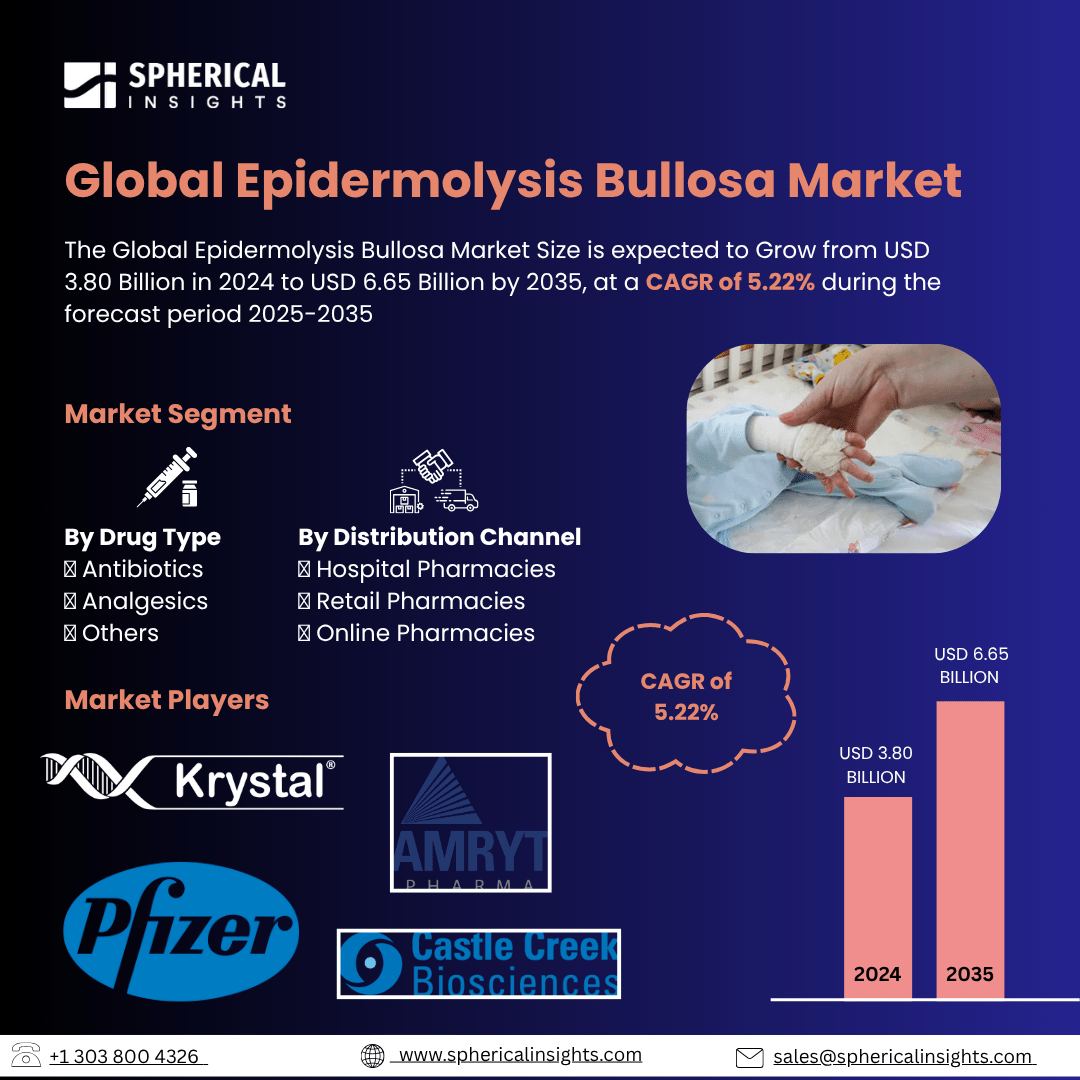
- As per Spherical Insights & Consulting, The Global Epidermolysis Bullosa Market Size is expected to Grow from USD 3.80 Billion in 2024 to USD 6.65 Billion by 2035, at a CAGR of 5.22% during the forecast period 2025-2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Epidermolysis Bullosa Market Companies such as Krystal Biotech, Amryt Pharma, Pfizer, Castle Creek Biosciences, Ultragenyx Pharmaceutical, AnaptysBio, Regeneron Pharmaceuticals, Amicus Therapeutics, Genzyme, Mallinckrodt Pharmaceuticals, BioMarin Pharmaceutical, Abeona Therapeutics, Argenx, Intrexon Corporation, and Others.
-
Epidermolysis Bullosa Treatment Market: Understanding and Treatment Algorithm:
Epidermolysis Bullosa (EB) is a rare genetic disorder causing extremely fragile skin that blisters and tears easily from minor friction or trauma. It affects the skin and mucous membranes, leading to painful wounds, risk of infection, and impaired healing, significantly impacting patients’ quality of life and requiring specialized care.
Epidermolysis Bullosa Diagnosis:
Epidermolysis Bullosa diagnosis involves clinical evaluation of skin fragility and blistering, supported by skin biopsy and immunofluorescence to identify specific protein defects. Genetic testing confirms mutations in genes like COL7A1, helping classify EB subtypes for accurate prognosis and personalized treatment planning.
Epidermolysis Bullosa Treatment
Treatment focuses on wound care, pain management, infection prevention, and improving skin healing. Emerging therapies include gene and cell therapies targeting the underlying genetic defects. Supportive care involves specialized dressings, nutritional support, and physical therapy to enhance quality of life and reduce complications in patients.
Epidermolysis Bullosa Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Epidermolysis Bullosa, Gender-specific Diagnosed Incidence of Epidermolysis Bullosa, Type-specific Diagnosed Incidence of Epidermolysis Bullosa, Age-specific Diagnosed Incidence of Epidermolysis Bullosa, Diagnosed Incident Population based on Primary Site of Epidermolysis Bullosa, and Diagnosed Incident Population based on Histologic Classification of Epidermolysis Bullosa Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of epidermolysis bullosa epidemiology in major markets worldwide.
Country Wise- Epidermolysis Bullosa Multiforme Epidemiology
- The epidemiology segment provides Epidermolysis Bullosa prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
Epidermolysis Bullosa Recent Developments:
- In May 2023, Krystal Biotech announced that the FDA approved VYJUVEK™ (beremagene geperpavec-svdt) for treating patients six months or older with dystrophic epidermolysis bullosa (DEB). VYJUVEK, the first-ever redosable gene therapy, delivers functional COL7A1 gene copies to promote wound healing and sustained protein expression.
Epidermolysis Bullosa Marketed Drugs:
VYJUVEK (beremagene geperpavec-svdt) is a gene therapy designed to deliver functional copies of the COL7A1 gene to patients with dystrophic epidermolysis bullosa (DEB). It is FDA-approved as the first redosable gene therapy aimed at promoting wound healing by restoring collagen VII protein expression.
- Zevaskyn: Abeona Therapeutics
Zevaskyn (pz-cel) is an autologous cell-based gene therapy approved for recessive dystrophic epidermolysis bullosa. It involves transplanting genetically corrected skin cells to promote healing of chronic wounds and reduce blister formation.
- Oleogel-S10: Amryt Pharma
Oleogel-S10 is a topical gel containing birch bark extract that promotes wound healing in EB patients. It is used to reduce blistering and improve skin regeneration in dystrophic EB cases.
Epidermolysis Bullosa: Emerging Therapies
- BNT327: BNT327 is a bispecific antibody in late-stage trials for EB. It targets two immune checkpoints to boost T-cell activation, aiming to enhance skin repair and reduce inflammation in patients with severe disease forms.
- SYNB-1010: SYNB-1010 is an oral engineered probiotic therapy designed to modulate gut microbiota and reduce systemic inflammation, potentially improving skin integrity and wound healing in EB patients.
- Sangamo’s SB-525: SB-525 is a gene editing therapy in development targeting mutations in the COL7A1 gene responsible for dystrophic EB. It aims to correct the genetic defect at the DNA level, offering a potential long-term cure.
- RGN-137: RGN-137 is a topical synthetic peptide that promotes wound healing by stimulating natural repair mechanisms and reducing inflammation. It is being investigated for treating chronic wounds in EB patients.
Epidermolysis Bullosa Market Outlook
- The epidermolysis bullosa market refers to the development and commercialization of treatments and diagnostics for a rare genetic skin disorder causing fragile, blister-prone skin. It includes gene therapies, topical treatments, and wound care products aimed at managing symptoms and improving patients’ quality of life globally.
- Key drivers of the epidermolysis bullosa market include increasing prevalence of EB, advancements in gene and cell therapies, growing awareness, rising healthcare expenditure, and expanding research investments. Additionally, improved diagnostic technologies and unmet medical needs for effective treatments are accelerating market growth worldwide.
- Opportunities in the market include the development of innovative gene and cell therapies, expansion into emerging markets, increased government funding, and collaborations for research. Growing patient awareness and advancements in personalized medicine also offer significant potential for new treatment options and market growth.
- Governments support epidermolysis bullosa through funding research, approving innovative therapies like Zevaskyn, and promoting awareness programs. Organizations collaborate with agencies to improve patient care and drive policy changes worldwide.
- Challenges include limited treatment options, high therapy costs, complex disease management, and low patient awareness in the epidermolysis bullosa market.
- The market is projected to grow due to increasing EB prevalence and advancements in gene and cell therapies.
Epidermolysis Bullosa Market Segmentation
By Drug Type:
- Antibiotics
- Analgesics
- Others
The Antibiotics segment dominates the Global Epidermolysis Bullosa market due to the high risk of skin infections caused by frequent blistering and wounds. Preventing and treating infections is critical to managing complications, making antibiotics essential and widely used, thereby capturing the largest market share among drug types.
By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
The Hospital Pharmacies segment dominates the Epidermolysis Bullosa market due to the need for specialized treatments and careful monitoring of patients. Hospitals provide access to advanced therapies, wound care products, and professional medical support, making them the primary distribution channel for managing this complex and severe skin disorder.
Regional Segment Analysis of the Epidermolysis Bullosa Market
North America holds the largest share in the epidermolysis bullosa market due to its advanced healthcare infrastructure, strong presence of key pharmaceutical companies, and high awareness of rare genetic disorders. The region benefits from significant R&D investments, early adoption of innovative therapies like gene and cell treatments, and robust reimbursement policies. Additionally, regulatory support from agencies such as the FDA facilitates faster approval and market access of novel EB therapies, driving market dominance.
The Asia-Pacific region is the fastest-growing market for epidermolysis bullosa, fueled by increasing healthcare expenditure, rising disease awareness, and improving diagnostic capabilities. Growing government initiatives to support rare disease treatment and expanding healthcare infrastructure contribute to rapid market expansion. Emerging economies such as China and India offer a large patient pool and increasing access to advanced therapies, while collaborations between local and global companies enhance market penetration and innovation.
Epidermolysis Bullosa Market Key Companies
- Krystal Biotech
- Amryt Pharma
- Pfizer
- Castle Creek Biosciences
- Ultragenyx Pharmaceutical
- AnaptysBio
- Regeneron Pharmaceuticals
- Amicus Therapeutics
- Genzyme
- Mallinckrodt Pharmaceuticals
- BioMarin Pharmaceutical
- Abeona Therapeutics
- Argenx
- Intrexon Corporation
- Others
Epidermolysis Bullosa Therapeutics Market Report Scope
- The Epidermolysis Bullosa therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Epidermolysis Bullosa’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Epidermolysis Bullosa therapies is provided, including an evaluation of new treatments expected to influence the current Epidermolysis Bullosa treatment market landscape.
- The report includes a detailed review of the Epidermolysis Bullosa therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient-Based Epidermolysis Bullosa Market Forecasting report offers valuable insights into trends shaping the global Epidermolysis Bullosa market, helping to develop effective business strategies.
Epidermolysis Bullosa Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Epidermolysis Bullosa Therapeutic Approaches in Epidermolysis Bullosa
- Review Of Drugs in Development for Epidermolysis Bullosa
- Market, Growth, and Trends in Epidermolysis Bullosa
- Market Opportunities in Epidermolysis Bullosa Treatment
- Effects Of Future Therapies on Epidermolysis Bullosa Treatment.
Epidermolysis Bullosa Treatment Market Report Key Strengths
- 15 Years Epidermolysis Bullosa Market Forecast
- Global Coverage
- Epidermolysis Bullosa Epidemiology Segmentation
- Key Cross Competition
Epidermolysis Bullosa Treatment Market Report Assessment
- Present Practices in the Epidermolysis Bullosa Treatment Market
- Review of Investigational Epidermolysis Bullosa Drugs
- Attractiveness of the Epidermolysis Bullosa Drug Market
- Epidermolysis Bullosa Market Drivers
- Epidermolysis Bullosa Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the epidermolysis bullosa market based on the below-mentioned segments:
Global Epidermolysis Bullosa Market, By Drug Type
- Antibiotics
- Analgesics
- Others
Global Epidermolysis Bullosa Market, By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Global Epidermolysis Bullosa Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa