Hereditary Angioedema Treatment Market: Understanding and Treatment Algorithm:
Hereditary angioedema (HAE) is a rare genetic disorder causing sudden, severe swelling in the skin, airways, and internal organs. It results from a deficiency or dysfunction of the C1 esterase inhibitor protein, leading to excessive bradykinin production. Symptoms can be life threatening, requiring timely diagnosis and treatment.
Hereditary Angioedema Diagnosis:
Diagnosis of hereditary angioedema involves evaluating patient history, physical symptoms, and family history. Laboratory tests measure levels and function of C1-esterase inhibitor and complement proteins. Genetic testing may confirm mutations. Accurate diagnosis is essential for effective treatment and to distinguish HAE from other causes of swelling.
Hereditary Angioedema Treatment:
Treatment for hereditary angioedema includes on-demand therapies to manage acute attacks and prophylactic treatments to prevent episodes. Options include C1-esterase inhibitors, bradykinin receptor antagonists, and kallikrein inhibitors. Therapy choice depends on attack frequency, severity, and patient needs, aiming to reduce symptoms and improve quality of life.
Hereditary Angioedema Therapeutics Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Hereditary Angioedema Therapeutics, Gender-specific Diagnosed Incidence of Hereditary Angioedema Therapeutics, Type specific Diagnosed Incidence of Hereditary Angioedema Therapeutics, Age-specific Diagnosed Incidence of Hereditary Angioedema Therapeutics, Diagnosed Incident Population based on Primary Site of Hereditary Angioedema Therapeutics, and Diagnosed Incident Population based on Histologic Classification of Hereditary Angioedema Therapeutics Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of hereditary angioedema therapeutics epidemiology in major markets worldwide.
Country Wise Hereditary Angioedema Therapeutics Multiforme Epidemiology
- The epidemiology segment provides Hereditary Angioedema Therapeutics prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia Pacific (including Japan), Latin America, the Middle East, and Africa.
Hereditary Angioedema Therapeutics: Recent Developments:
- In July 2025, KalVista Pharmaceuticals announced the U.S. FDA approval of Ekterly (sebetralstat), the first and only oral on demand treatment for hereditary angioedema (HAE). This approval marks a significant advancement in HAE management, offering patients a convenient alternative to injectable therapies.
Hereditary Angioedema Therapeutics Marketed Drugs:
Berinert is a plasma-derived C1esterase inhibitor used for the treatment and prevention of hereditary angioedema attacks. It works by replacing the deficient or dysfunctional C1 inhibitor protein, helping to control swelling episodes effectively. It is FDA approved for both acute treatment and short-term prophylaxis.
- Firazyr (Icatibant): Shire (Takeda)
Firazyr is a bradykinin B2 receptor antagonist administered via subcutaneous injection. It blocks bradykinin receptors, reducing swelling during acute hereditary angioedema attacks. Firazyr is approved for on-demand treatment of HAE attacks.
- Kalbitor (Ecallantide): Dyax (Shire/Takeda)
Kalbitor is a kallikrein inhibitor that prevents the generation of bradykinin, thereby reducing the severity of HAE attacks. It is given as a subcutaneous injection for acute treatment and is FDA-approved for this use.
Hereditary Angioedema Therapeutics: Emerging Therapies
- BNT327: It is a bispecific antibody in late-stage trials targeting two immune checkpoints to enhance immune regulation. Though primarily developed for oncology, its immune-modulating potential is being explored for improving outcomes in patients with hereditary angioedema resistant to conventional treatments.
- Garadacimab: It is a monoclonal antibody targeting activated Factor XIIa, which plays a key role in HAE attacks. It is being investigated as a prophylactic treatment to reduce the frequency and severity of swelling episodes.
- Donidalorsen: It is an antisense oligonucleotide designed to reduce plasma prekallikrein production, lowering bradykinin levels to prevent HAE attacks. It offers a promising prophylactic approach with potential for less frequent dosing.
- Veldoceran: It is a novel oral kallikrein inhibitor under clinical development, aiming to provide a convenient, non-invasive treatment option for HAE patients, improving adherence and quality of life.
Hereditary Angioedema Therapeutics Market Outlook
- The Hereditary Angioedema Therapeutics Market involves drugs and treatments aimed at preventing and managing hereditary angioedema, a rare genetic disorder causing sudden swelling attacks. Therapies include C1 esterase inhibitors, bradykinin receptor antagonists, and kallikrein inhibitors to control symptoms and improve patient quality of life.
- Key drivers include rising awareness of hereditary angioedema, increasing diagnosis rates, and advancements in targeted therapies. Growing patient populations and improved healthcare infrastructure globally have enhanced access to treatment. Additionally, innovation in biologics and expanding therapeutic options fuel market growth.
- Emerging markets present significant growth opportunities due to unmet medical needs and increasing healthcare spending. Ongoing research in gene therapy and novel biologics offers potential breakthroughs. Furthermore, expanding patient awareness and supportive reimbursement policies in developing countries can drive broader market adoption.
- Governments worldwide are implementing rare disease policies, funding research, and supporting access to orphan drugs. Increased insurance coverage and patient assistance programs improve treatment affordability. Public health campaigns focus on early diagnosis and education, facilitating the timely management of hereditary angioedema.
- High treatment costs and limited patient awareness in certain regions hinder market penetration.
- A major challenge in the Hereditary Angioedema Therapeutics market is the limited effectiveness of treatments in advanced stages and the high recurrence rate despite therapy.
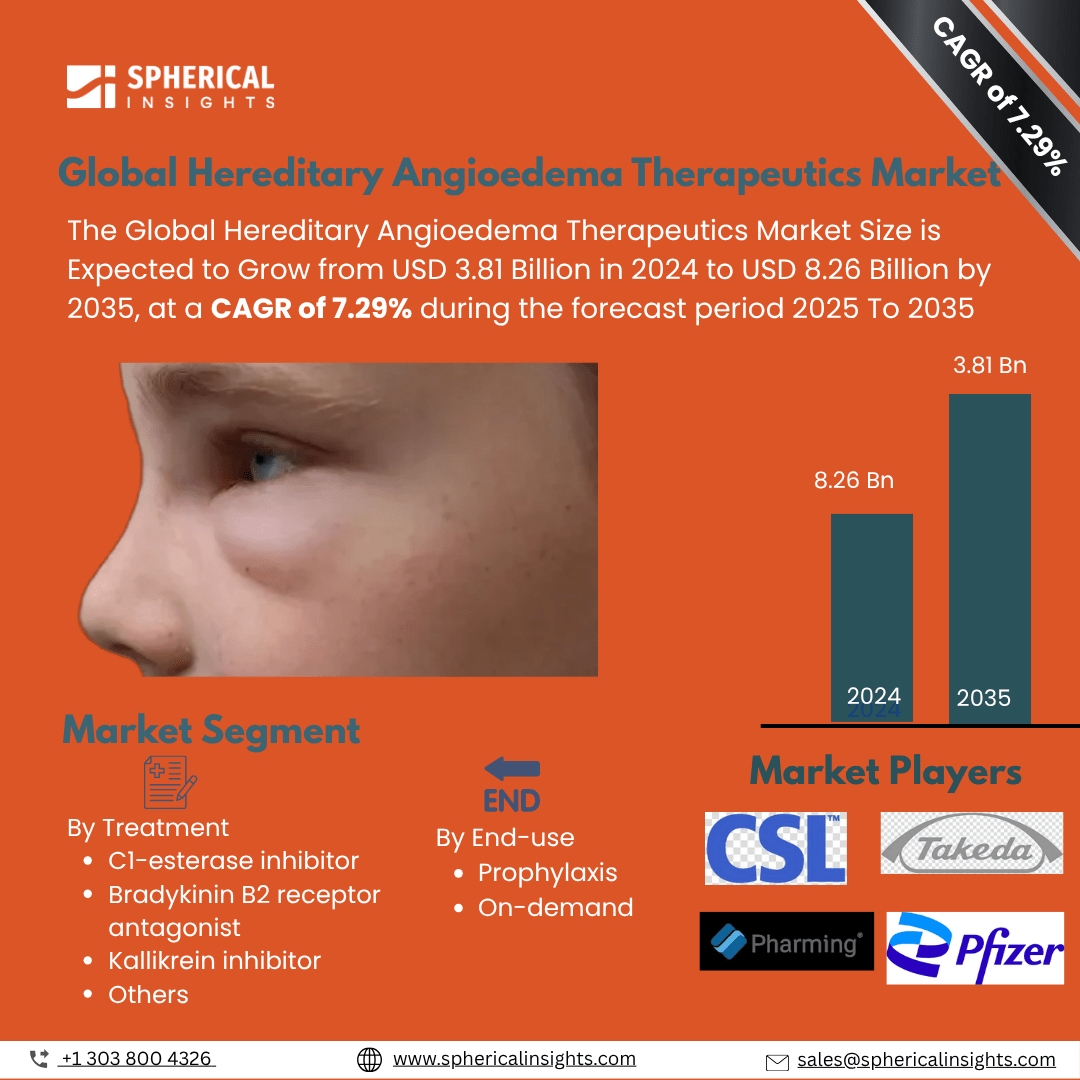
- The market is projected to grow due to increasing diagnoses and availability of innovative, targeted therapies, improving patient outcomes.
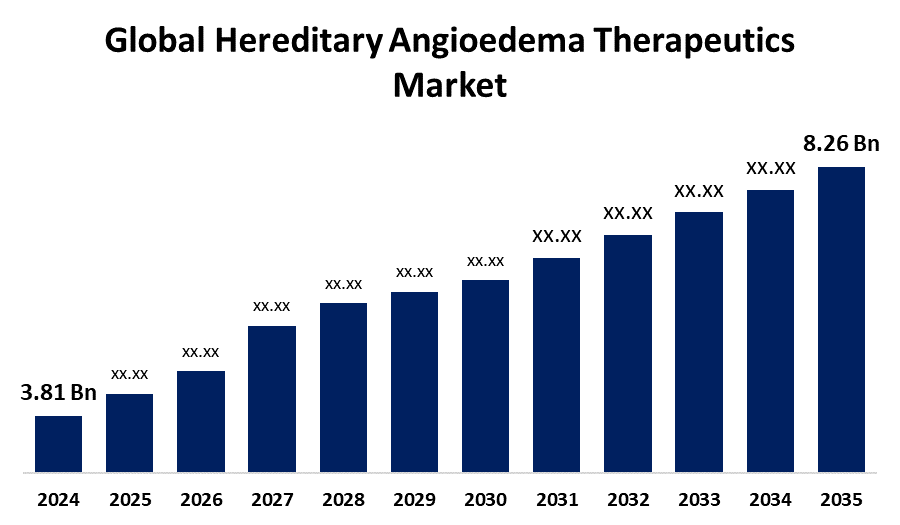
Hereditary Angioedema Therapeutics Market Segmentation
By Treatment:
- C1 Esterase Inhibitor
- Bradykinin B2 Receptor Antagonist
- Kallikrein Inhibitor
- Others
The C1-esterase inhibitor segment holds the largest share in the hereditary angioedema (HAE) therapeutics market. It is the most established and widely used treatment, offering both prophylactic and on-demand relief. High efficacy, availability in various forms (IV, SC), and regulatory approvals support its widespread clinical adoption and market dominance.
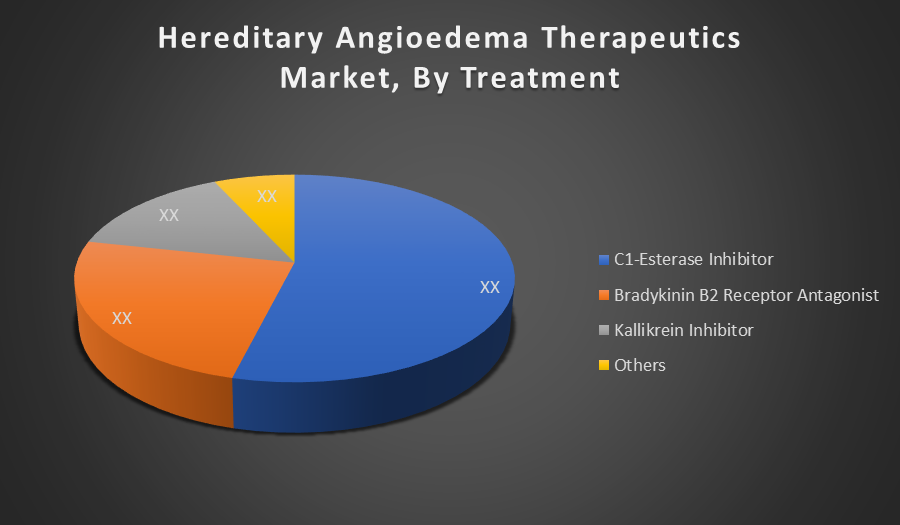
By End-use:
The On-Demand segment leads the market due to the urgent nature of HAE attacks, requiring immediate intervention. Most patients rely on fast-acting treatments during unpredictable episodes to prevent life-threatening complications. This has driven consistent demand, especially for injectable therapies, ensuring a dominant position over prophylactic use in the overall market share.
Regional Segment Analysis of the Hereditary Angioedema Therapeutics Market
North America holds the largest share in the hereditary angioedema therapeutics market due to its advanced healthcare infrastructure, strong presence of key pharmaceutical companies, and high awareness among patients and healthcare providers. Well-established reimbursement systems and early adoption of innovative therapies also contribute to the region’s dominance. Additionally, active research and development efforts and favourable regulatory frameworks support continuous market growth in this region.
Asia-Pacific is the fastest-growing region in the hereditary angioedema therapeutics market, driven by increasing disease awareness and improving healthcare infrastructure. Rising healthcare expenditure and expanding access to advanced therapies are fueling growth. Furthermore, growing patient populations and increasing diagnosis rates contribute to market expansion. Governments’ focus on rare disease management and supportive policies in emerging economies is also accelerating the adoption of HAE treatments in this region.
Hereditary Angioedema Therapeutics Market Key Companies
- CSL Behring
- Takeda Pharmaceutical
- Shire
- BioCryst Pharmaceuticals
- Pharming Group
- Pfizer
- Sanofi
- Dyax Corporation
- Ferring Pharmaceuticals
- KalVista Pharmaceuticals
- Janssen Pharmaceuticals
- Regeneron Pharmaceuticals
- Octapharma
- Apellis Pharmaceuticals
- Others
Hereditary Angioedema Therapeutics Therapeutics Market Report Scope
- The Hereditary Angioedema Therapeutics therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Hereditary Angioedema Therapeutics’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Hereditary Angioedema Therapeutics therapies is provided, including an evaluation of new treatments expected to influence the current Hereditary Angioedema Therapeutics treatment market landscape.
- The report includes a detailed review of the Hereditary Angioedema Therapeutics therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient-Based Hereditary Angioedema Therapeutics Market Forecasting report offers valuable insights into trends shaping the global Hereditary Angioedema Therapeutics market, helping to develop effective business strategies.
Hereditary Angioedema Therapeutics Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Hereditary Angioedema Therapeutics Therapeutic Approaches in Hereditary Angioedema Therapeutics
- Review Of Drugs in Development for Hereditary Angioedema Therapeutics
- Market, Growth, and Trends in Hereditary Angioedema Therapeutics
- Market Opportunities in Hereditary Angioedema Therapeutics Treatment
- Effects Of Future Therapies on Hereditary Angioedema Therapeutics Treatment.
Hereditary Angioedema Therapeutics Treatment Market Report Key Strengths
- 15 Years Hereditary Angioedema Therapeutics Market Forecast
- Global Coverage
- Hereditary Angioedema Therapeutics Epidemiology Segmentation
- Key Cross Competition
Hereditary Angioedema Therapeutics Treatment Market Report Assessment
- Present Practices in the Hereditary Angioedema Therapeutics Treatment Market
- Review of Investigational Hereditary Angioedema Therapeutics Drugs
- Attractiveness of the Hereditary Angioedema Therapeutics Drug Market
- Hereditary Angioedema Therapeutics Market Drivers
- Hereditary Angioedema Therapeutics Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the hereditary angioedema therapeutics market based on the below-mentioned segments:
Global Hereditary Angioedema Therapeutics Market, By Treatment
- C1-esterase inhibitor
- Bradykinin B2 receptor antagonist
- Kallikrein inhibitor
- Others
Global Hereditary Angioedema Therapeutics Market, By End-use
Global Hereditary Angioedema Therapeutics Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East Africa