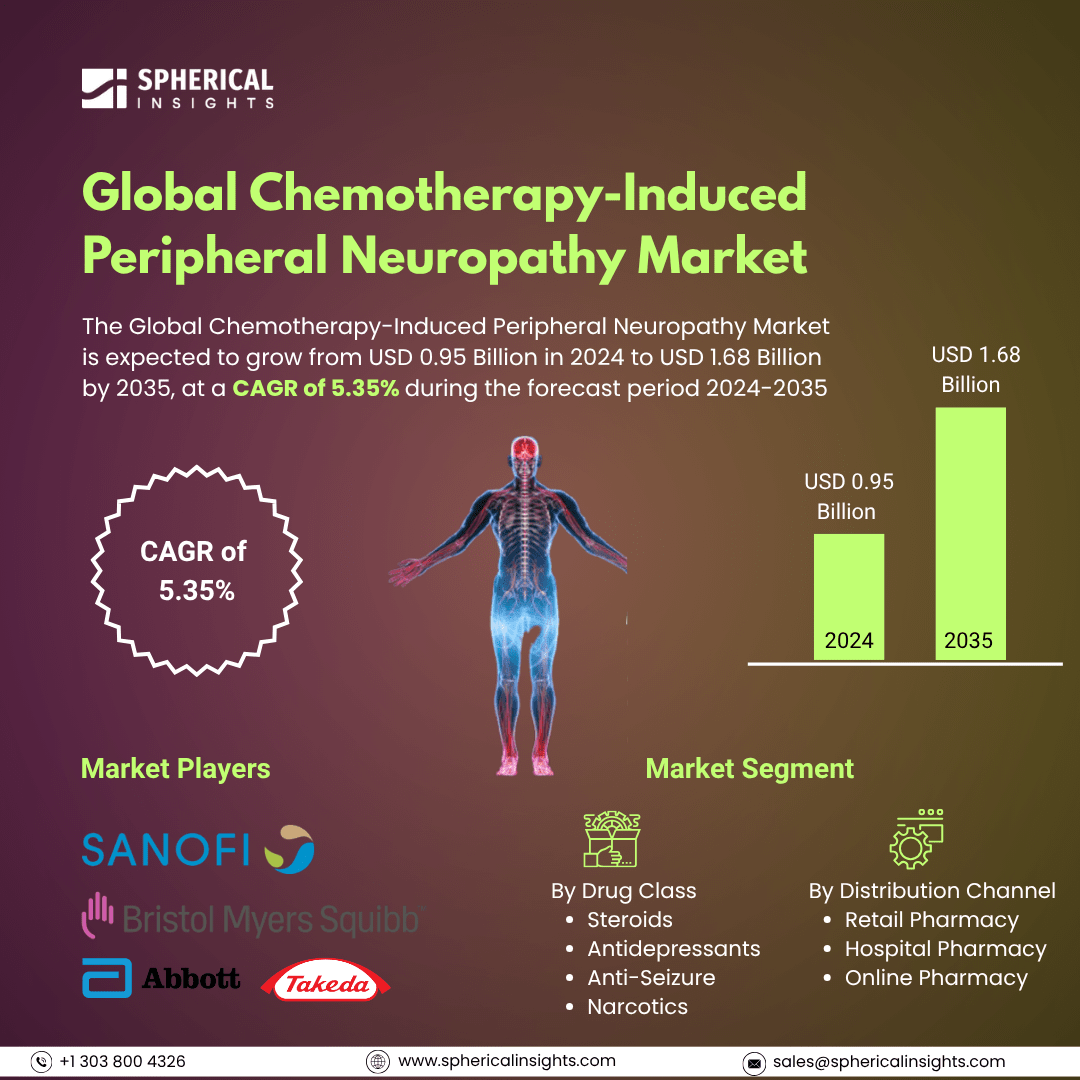
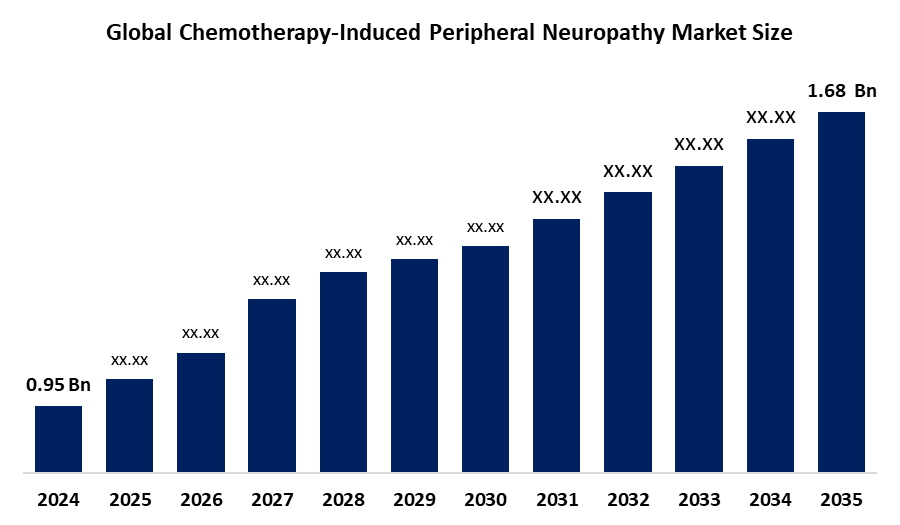
- As per Spherical Insights & Consulting, The Global Chemotherapy-Induced Peripheral Neuropathy Market Size is Expected to Grow from USD 0.95 Billion in 2024 to USD 1.68 Billion by 2035, at a CAGR of 5.35% during the forecast period 2024-2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- In 2024, The United States held the largest market share for Chemotherapy-Induced Peripheral Neuropathy, surpassing that of the EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- The leading Chemotherapy-Induced Peripheral Neuropathy Market Companies are Sanofi SA, Bristol Myers Squibb, Abbott Laboratories, Takeda, Amgen, Asahi Kasei Pharma, Genentech (Roche), Jazz Pharmaceuticals, NeuroMetrix, MediciNova, ChromaDex, WEX Pharmaceuticals, Solasia Pharma, Nemus Bioscience, Aphios, Kineta, Aptinyx, Apexian Pharma, Krenitsky Pharmaceuticals, WinSanTor Inc., and Others.
Chemotherapy-Induced Peripheral Neuropathy Treatment Market: Understanding and Treatment Algorithm:
Chemotherapy-Induced Peripheral Neuropathy (CIPN) is a common side effect of cancer treatments, causing pain, numbness, and tingling in the hands and feet. Current treatment options are limited, with duloxetine being the only recommended drug. Ongoing research focuses on developing novel therapies to prevent or alleviate symptoms, aiming to improve patient quality of life and treatment adherence.
Chemotherapy-Induced Peripheral Neuropathy Diagnosis:
Chemotherapy-induced peripheral Neuropathy (CIPN) diagnosis involves clinical evaluation of symptoms like numbness, tingling, and pain in the extremities during or after chemotherapy. Physicians use patient history, neurological exams, and scales like the Total Neuropathy Score. Early detection is crucial to adjusting treatment plans, preventing progression, and reducing long-term nerve damage associated with neurotoxic chemotherapy agents.
Chemotherapy-Induced Peripheral Neuropathy Treatment
Chemotherapy-Induced Peripheral Neuropathy (CIPN) treatment focuses on symptom management, as no cure currently exists. Duloxetine is the only recommended medication for CIPN-related pain. Other options include anticonvulsants, antidepressants, topical agents, and non-pharmacological therapies like acupuncture and physical therapy. Ongoing research aims to develop more effective, targeted treatments to prevent or reverse nerve damage caused by chemotherapy.
Chemotherapy-Induced Peripheral Neuropathy Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Chemotherapy-Induced Peripheral Neuropathy, Gender-specific Diagnosed Incidence of Chemotherapy-Induced Peripheral Neuropathy, Type-specific Diagnosed Incidence of Chemotherapy-Induced Peripheral Neuropathy, Age-specific Diagnosed Incidence of Chemotherapy-Induced Peripheral Neuropathy, Diagnosed Incident Population based on Primary Site of Chemotherapy-Induced Peripheral Neuropathy, and Diagnosed Incident Population based on Histologic Classification of Chemotherapy-Induced Peripheral Neuropathy Tumor in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of Chemotherapy-Induced Peripheral Neuropathy epidemiology in major markets worldwide.
Country Wise- Chemotherapy-Induced Peripheral Neuropathy Multiforme Epidemiology
- The epidemiology segment provides Chemotherapy-Induced Peripheral Neuropathy prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
- In 2017, the United States recorded 173,683 cases of mild, 305,443 cases of moderate, and 119,782 cases of severe Chemotherapy-Induced Peripheral Neuropathy (CIPN). According to analysts, moderate CIPN had the highest incidence, followed by mild and severe cases. This trend is expected to rise significantly in the coming years due to increasing chemotherapy use.
- Among European countries in 2017, Germany had the highest number of new CIPN cases with 165,095, followed by the United Kingdom with 120,624 cases. In contrast, Spain reported the lowest incidence of CIPN, with 73,071 cases of CIPN during the same year.
- According to the publisher's estimates, Japan accounted for approximately 18.11% of the total incident cases of CIPN. In 2017, the incident population of CIPN in Japan was estimated to be 263,842.
Chemotherapy-Induced Peripheral Neuropathy Recent Developments:
- In January 2022, NeuroMetrix announced that its wearable device, Quell, received FDA Breakthrough Device Designation in 2022 for treating chronic Chemotherapy-Induced Peripheral Neuropathy (CIPN). In Phase 2 trials, Quell significantly reduced symptoms like burning and tingling, offering a promising non-invasive treatment for persistent CIPN pain.
Chemotherapy-Induced Peripheral Neuropathy Marketed Drugs:
- Duloxetine: Eli Lilly and Company
Duloxetine is a serotonin-norepinephrine reuptake inhibitor (SNRI) that works by increasing the levels of neurotransmitters serotonin and norepinephrine in the central nervous system, helping to modulate pain signals. It is FDA-approved for the treatment of neuropathic pain, including Chemotherapy-Induced Peripheral Neuropathy (CIPN). Duloxetine alleviates symptoms such as burning, tingling, and numbness by targeting nerve pain pathways. In 2013, the US FDA approved duloxetine for managing neuropathic pain associated with diabetic peripheral neuropathy and later it became widely used off-label for CIPN symptom relief.
Pregabalin is an anticonvulsant and neuropathic pain agent that works by binding to the α2δ subunit of voltage-gated calcium channels in the central nervous system, which reduces the release of excitatory neurotransmitters and decreases nerve pain signaling. It is approved by the FDA for neuropathic pain conditions such as diabetic peripheral neuropathy and postherpetic neuralgia. While not specifically approved for Chemotherapy-Induced Peripheral Neuropathy (CIPN), pregabalin is commonly prescribed off-label to help manage CIPN symptoms like burning, tingling, and numbness in patients undergoing chemotherapy.
Venlafaxine is a serotonin-norepinephrine reuptake inhibitor (SNRI) marketed by Pfizer. It works by increasing serotonin and norepinephrine levels in the brain to help regulate mood and pain perception. Venlafaxine is FDA-approved for major depressive disorder, generalized anxiety disorder, and panic disorder, but is not specifically approved for Chemotherapy-Induced Peripheral Neuropathy (CIPN). However, it is sometimes prescribed off-label to alleviate neuropathic pain symptoms associated with CIPN, such as burning and tingling sensations.
Gabapentin is an anticonvulsant and neuropathic pain medication originally marketed by Pfizer. It modulates calcium channels in the nervous system to reduce abnormal nerve activity and alleviate pain. Gabapentin is FDA-approved for the treatment of seizures and postherpetic neuralgia but is not specifically approved for Chemotherapy-Induced Peripheral Neuropathy (CIPN). Despite this, it is widely used off-label to manage CIPN symptoms such as nerve pain, burning, and tingling.
Chemotherapy-Induced Peripheral Neuropathy Emerging therapies
- ART26.12: It is a selective FABP5 inhibitor that boosts endocannabinoid levels to reduce neuropathic pain. It shows promise in preclinical studies for treating Chemotherapy-Induced Peripheral Neuropathy by alleviating symptoms like hyperalgesia and allodynia through modulation of pain pathways.
- Ibudilast (MN-166): By MediciNova, is an anti-inflammatory and neuroprotective agent under clinical investigation. It aims to reduce nerve inflammation and improve sensory function in CIPN patients, offering a potential therapeutic option to manage chemotherapy-induced nerve damage and related pain.
- Halneuron (tetrodotoxin): By WEX Pharmaceuticals, is a sodium channel blocker in Phase II trials. It targets nerve excitability to reduce neuropathic pain in CIPN patients, showing promise as a novel treatment to alleviate persistent chemotherapy-induced nerve pain symptoms.
- Quell: It is developed by NeuroMetrix, a wearable neurostimulation device granted FDA Breakthrough Device Designation. It delivers transcutaneous electrical nerve stimulation to relieve chronic CIPN pain, providing a non-invasive option for patients with persistent chemotherapy-induced neuropathy symptoms.
Chemotherapy-Induced Peripheral Neuropathy Market Outlook
- The Chemotherapy-Induced Peripheral Neuropathy market encompasses the diagnosis, treatment, and management of peripheral nerve damage caused by chemotherapeutic agents. This includes pharmaceutical drugs, medical devices, and supportive therapies aimed at alleviating pain, numbness, tingling, and motor dysfunction in cancer patients undergoing or following chemotherapy. The market also covers emerging therapies, clinical trials, and innovations focused on prevention, symptom control, and nerve regeneration related to CIPN.
- The CIPN market is driven by the rising global incidence of cancer and increased use of neurotoxic chemotherapeutic agents such as taxanes, platinum-based drugs, and vinca alkaloids. Additionally, growing awareness, unmet medical needs, and ongoing advancements in neuroprotective and symptomatic treatment options are fueling market growth.
- The CIPN market presents strong opportunities through the development of targeted, non-opioid therapies, increased investment in clinical research, and growing adoption of wearable pain management devices. Expanding oncology treatment populations and unmet needs also create space for innovative therapeutic solutions.
- Government initiatives supporting the CIPN market include FDA guidance for drug development and funding by the National Cancer Institute for clinical trials focused on CIPN prevention, treatment, and patient-centered research outcomes.
- A major challenge in the CIPN market is the lack of FDA-approved, targeted therapies. Current treatments offer limited efficacy, and variability in patient response makes it difficult to develop standardized, effective interventions.
- The CIPN market is projected to grow significantly over the coming years, driven by increasing cancer prevalence and advancements in targeted therapies and supportive care solutions.
Chemotherapy-Induced Peripheral Neuropathy Market Segmentation
By Drug Class
- Steroids
- Antidepressants
- Anti-Seizure
- Narcotics
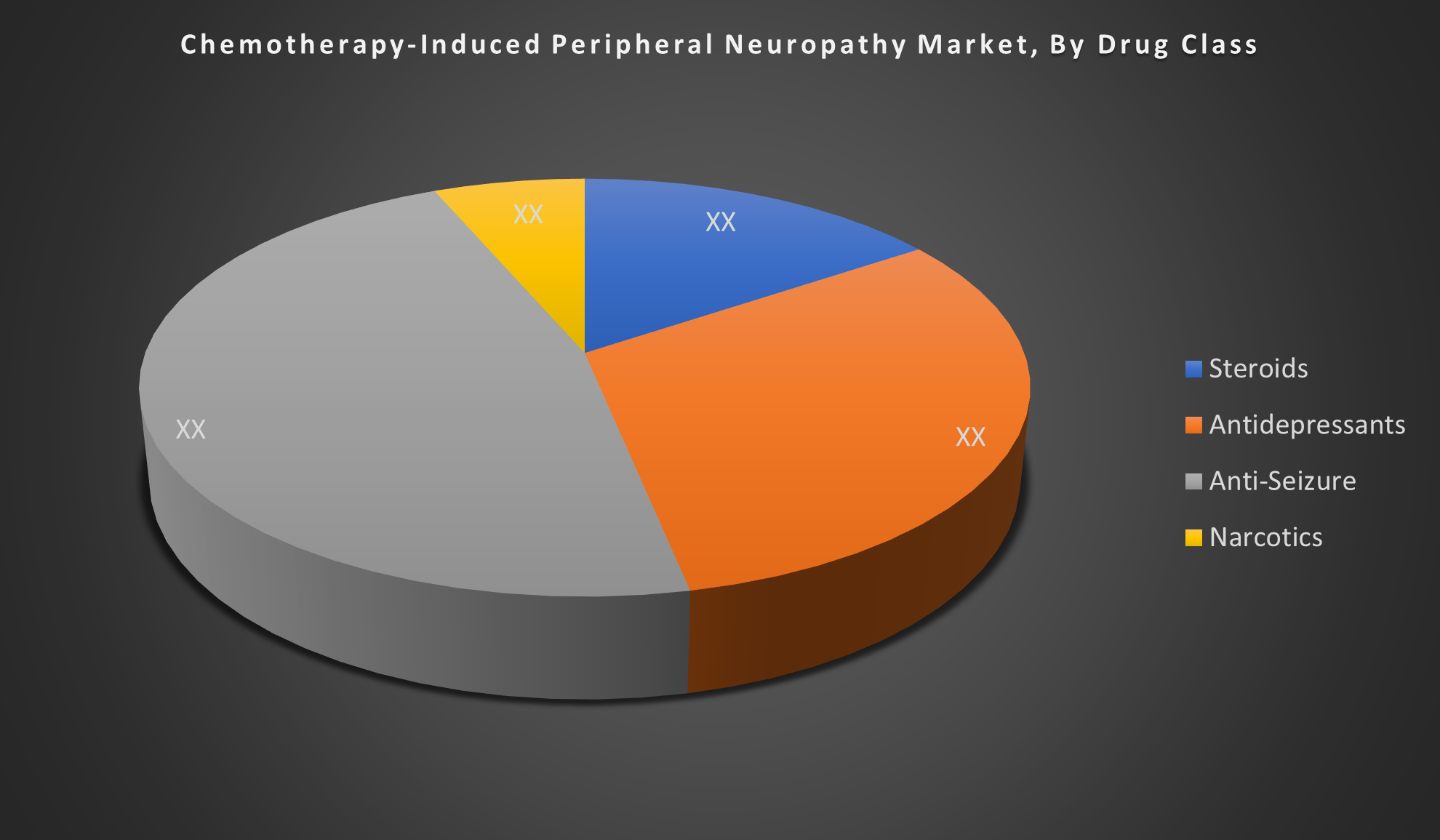
The Anti-Seizure segment dominates the global CIPN market due to the widespread use of drugs like gabapentin and pregabalin. These are preferred for their effectiveness in managing neuropathic pain, better patient tolerance, and broad off-label use, despite limited FDA-approved CIPN-specific treatments.
By Distribution Channel
- Retail Pharmacy
- Hospital Pharmacy
- Online Pharmacy
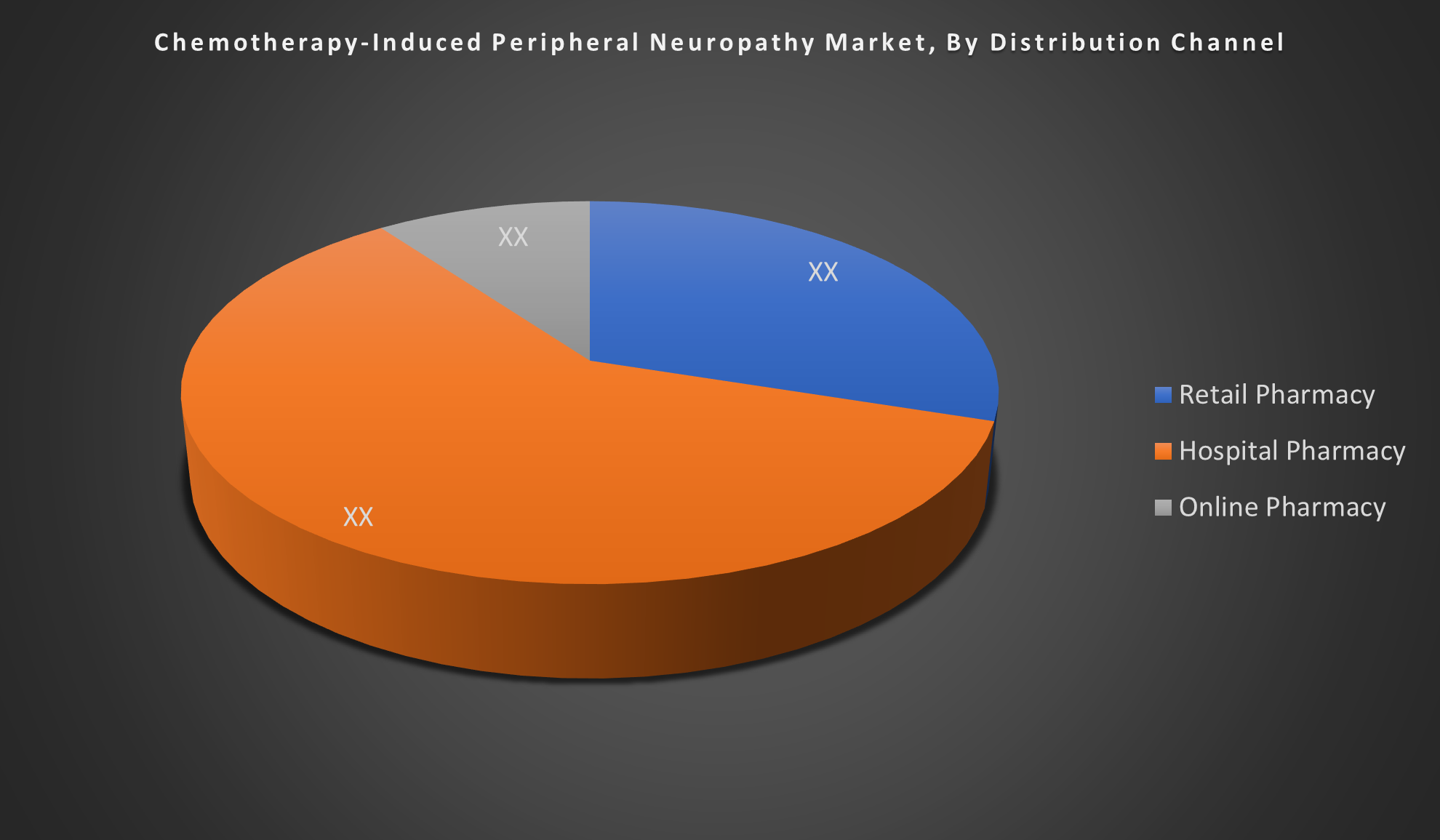
The Hospital Pharmacy segment dominates the global CIPN market due to its central role in chemotherapy administration and immediate medication access for managing symptoms. However, retail pharmacies are expected to grow fastest, driven by increasing outpatient treatments and patient preference for convenient medication access.
Regional Segment Analysis of the Chemotherapy-Induced Peripheral Neuropathy Market
North America leads the global Chemotherapy-Induced Peripheral Neuropathy (CIPN) market, with the United States playing a key role. The region’s dominance is driven by a high prevalence of cancer, well-established healthcare infrastructure, and significant investments in pharmaceutical research and development. Additionally, strong awareness among healthcare providers and patients about CIPN, along with access to advanced diagnostic and treatment options, supports market growth. The presence of leading pharmaceutical companies and ongoing clinical trials for new therapies further strengthens the market. Moreover, government initiatives focused on cancer care and patient support contribute to the robust demand for CIPN management solutions in North America.
The Asia-Pacific region is the fastest-growing market for Chemotherapy-Induced Peripheral Neuropathy (CIPN), fueled by rising cancer cases and expanding healthcare access in countries like China, India, and Japan. Increasing investments in oncology treatments and growing awareness among patients and healthcare providers are driving demand, making this region the most rapidly developing market globally.
Chemotherapy-Induced Peripheral Neuropathy Therapeutics Market Key Companies
- Sanofi SA
- Bristol Myers Squibb
- Abbott Laboratories
- Takeda
- Amgen
- Asahi Kasei Pharma
- Genentech (Roche)
- Jazz Pharmaceuticals
- NeuroMetrix
- MediciNova
- ChromaDex
- WEX Pharmaceuticals
- Solasia Pharma
- Nemus Bioscience
- Aphios
- Kineta
- Aptinyx
- Apexian Pharma
- Krenitsky Pharmaceuticals
- WinSanTor Inc.
- Others.
Chemotherapy-Induced Peripheral Neuropathy Therapeutics Market Report Scope
- The Chemotherapy-Induced Peripheral Neuropathy therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Chemotherapy-Induced Peripheral Neuropathy’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Chemotherapy-Induced Peripheral Neuropathy therapies is provided, including an evaluation of new treatments expected to influence the current Chemotherapy-Induced Peripheral Neuropathy treatment market landscape.
- The report includes a detailed review of the Chemotherapy-Induced Peripheral Neuropathy therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient-Based Chemotherapy-Induced Peripheral Neuropathy Market Forecasting report offers valuable insights into trends shaping the global Chemotherapy-Induced Peripheral Neuropathy market, helping to develop effective business strategies.
Chemotherapy-Induced Peripheral Neuropathy Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Chemotherapy-Induced Peripheral Neuropathy Therapeutic Approaches in Chemotherapy-Induced Peripheral Neuropathy
- Review Of Drugs in Development for Chemotherapy-Induced Peripheral Neuropathy
- Market, Growth, and Trends in Chemotherapy-Induced Peripheral Neuropathy
- Market Opportunities in Chemotherapy-Induced Peripheral Neuropathy Treatment
- Effects Of Future Therapies on Chemotherapy-Induced Peripheral Neuropathy Treatment.
Chemotherapy-Induced Peripheral Neuropathy Treatment Market Report Key Strengths
- 15 Years Chemotherapy-Induced Peripheral Neuropathy Market Forecast
- Global Coverage
- Chemotherapy-Induced Peripheral Neuropathy Epidemiology Segmentation
- Key Cross Competition
Chemotherapy-Induced Peripheral Neuropathy Treatment Market Report Assessment
- Present Practices in the Chemotherapy-Induced Peripheral Neuropathy Treatment Market
- Attractiveness of the Chemotherapy-Induced Peripheral Neuropathy Drug Market
- Chemotherapy-Induced Peripheral Neuropathy Market Drivers
- Chemotherapy-Induced Peripheral Neuropathy Market Barriers
- SWOT
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the chemotherapy-induced peripheral neuropathy market based on the below-mentioned segments:
Global Chemotherapy-Induced Peripheral Neuropathy Market, By Treatment Type
- Steroids
- Antidepressants
- Anti-Seizure
- Narcotics
Global Chemotherapy-Induced Peripheral Neuropathy Market, By Distribution Channel
- Retail Pharmacy
- Hospital Pharmacy
- Online Pharmacy
Global Chemotherapy-Induced Peripheral Neuropathy Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa