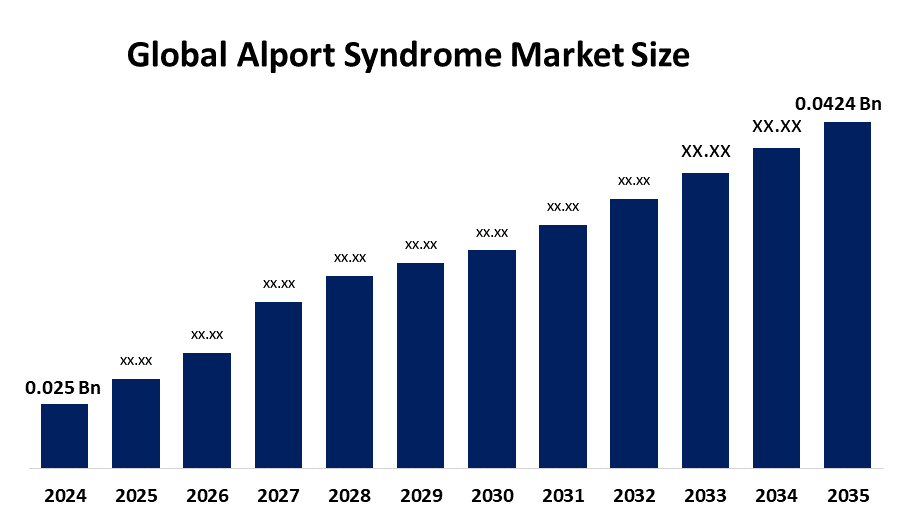
- As per Spherical Insights & Consulting, The Global Alport Syndrome Market Size is expected to grow from USD 0.025 Billion in 2024 to USD 0.0424 Billion by 2035, at a CAGR of 4.92% during the forecast period 2025-2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Alport Syndrome Market Companies such as AstraZeneca PLC, Boehringer Ingelheim International GmbH, Calliditas Therapeutics AB, CENTOGENE N.V., Chinook Therapeutics Inc., Daiichi Sankyo Company Limited, Eloxx Pharmaceuticals Inc., Eurofins Scientific SE, GlaxoSmithKline PLC, Illumina Inc., Invitae Corp., Merck & Co. Inc., Mylan N.V., Natera Inc., and Others.
Alport Syndrome Treatment Market: Understanding and Treatment Algorithm:
Alport Syndrome is a rare genetic disorder that affects the kidneys, ears, and eyes. It results from mutations in genes responsible for collagen production in the glomeruli. The condition leads to progressive kidney disease, hearing loss, and eye abnormalities, often requiring lifelong monitoring, supportive care, and, in severe cases, kidney transplantation.
Alport Syndrome Diagnosis:
Alport Syndrome is diagnosed through a combination of clinical evaluation, family history, and laboratory tests. Diagnostic methods include urine tests for protein and blood, kidney biopsy, hearing and eye exams, and genetic testing to confirm mutations in collagen-related genes. Early diagnosis is vital for managing progression and guiding treatment.
Alport Syndrome Treatment
Treatment focuses on slowing kidney damage and managing symptoms. It includes medications like ACE inhibitors or ARBs to control blood pressure and proteinuria, hearing aids for hearing loss, and regular monitoring. In advanced cases, dialysis or kidney transplantation may be necessary. Research into gene therapies is ongoing.
Alport Syndrome Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Alport Syndrome, Gender-specific Diagnosed Incidence of Alport Syndrome, Type-specific Diagnosed Incidence of Alport Syndrome, Age-specific Diagnosed Incidence of Alport Syndrome, Diagnosed Incident Population based on Primary Site of Alport Syndrome, and Diagnosed Incident Population based on Histologic Classification of Alport Syndrome Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of Alport Syndrome epidemiology in major markets worldwide.
Country Wise- Alport Syndrome Multiforme Epidemiology
- The epidemiology segment provides Alport Syndrome prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
Alport Syndrome Recent Developments:
- In November 2023, Calliditas Therapeutics AB announced the initiation of a Phase 2 clinical study to evaluate setanaxib in Alport syndrome. The randomized, double-blind, placebo-controlled trial aimed to assess safety, tolerability, and effects on UPCR and eGFR in 20 patients with proteinuria despite RAS blocker treatment over 24 weeks.
Alport Syndrome Marketed Drugs:
- Setanaxib: Calliditas Therapeutics AB
Setanaxib is a selective NOX1/4 inhibitor that reduces oxidative stress and fibrosis in kidney tissue. It is being developed for Alport Syndrome and has received orphan drug designation. The drug is in Phase 2 trials and targets kidney function preservation in affected patients.
- Bardoxolone Methyl: Reata Pharmaceuticals
Bardoxolone methyl is an oral Nrf2 activator that combats inflammation and oxidative stress in the kidneys. It has been studied in the Phase 3 CARDINAL trial for Alport Syndrome and has shown improvements in eGFR. It holds orphan drug status in both the U.S. and the EU.
- ELX-02: Eloxx Pharmaceuticals
ELX-02 is a synthetic compound that enables read-through of nonsense mutations in mRNA, restoring protein production in Alport Syndrome patients with specific genetic mutations. It is being investigated in clinical trials and has demonstrated potential to improve renal function.
Alport Syndrome: Emerging Therapies
- Setanaxib: Setanaxib is a selective NOX1/4 inhibitor in Phase 2 trials for Alport Syndrome. It targets kidney fibrosis and oxidative stress to slow disease progression and improve renal outcomes in patients with persistent proteinuria despite standard treatment.
- Bardoxolone Methyl: Bardoxolone methyl is an oral Nrf2 activator in late-stage trials for Alport Syndrome. It reduces inflammation and oxidative stress to enhance kidney function, aiming to delay the need for dialysis or transplantation in patients with declining eGFR.
- ELX-02: ELX-02 is a synthetic compound in clinical development for Alport Syndrome caused by nonsense mutations. It promotes read-through of premature stop codons to restore functional protein production and improve renal health in genetically defined patients.
- Vonafexor: Vonafexor is an FXR agonist in early-stage trials for Alport Syndrome. It modulates bile acid pathways to reduce renal inflammation and fibrosis, aiming to preserve kidney function and slow disease progression in affected individuals.
Alport Syndrome Market Outlook
- The Alport Syndrome market refers to the global landscape of diagnostics, treatments, and emerging therapies focused on managing this rare genetic kidney disorder, aiming to improve patient outcomes through early detection, innovative drugs, and clinical advancements.
- Key drivers of the Alport Syndrome market include rising awareness, improved genetic testing, increasing prevalence of rare kidney diseases, advancements in precision medicine, and strong research investments in targeted therapies aimed at slowing disease progression and enhancing patient quality of life.
- Opportunities in the Alport Syndrome market include the development of gene and mutation-specific therapies, expansion of newborn screening programs, increased orphan drug designations, collaborations between biotech firms, and growing demand for targeted treatments in rare kidney disorders across emerging healthcare markets
- Government initiatives in the Alport Syndrome market focus on funding rare disease research, supporting genetic screening programs, providing incentives for orphan drug development, and implementing policies to improve access to innovative therapies, aiming to enhance diagnosis and treatment for affected patients globally.
- Limited awareness and diagnosis difficulties hinder early detection and effective treatment of Alport Syndrome, challenging market growth.
- The Alport Syndrome market is projected to grow due to increasing disease awareness, advancements in genetic testing, development of targeted therapies, and rising investments in rare disease research.
Alport Syndrome Market Segmentation
By Genetic Mutations
- COL4A3 Mutations
- COL4A4 Mutations
- COL4A5 Mutations
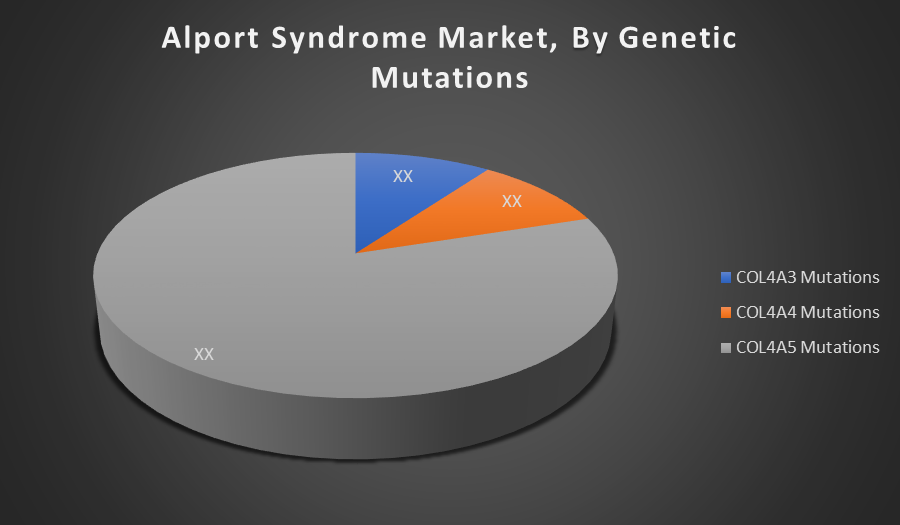
The COL4A5 Mutations segment holds the largest share due to its higher prevalence in X-linked Alport Syndrome, which is the most common form. This mutation leads to more severe symptoms, driving greater demand for diagnosis and targeted treatments.
By Severity:
- Alport Syndrome with Nephropathy
- Alport Syndrome without Nephropathy
- Ocular Alport Syndrome
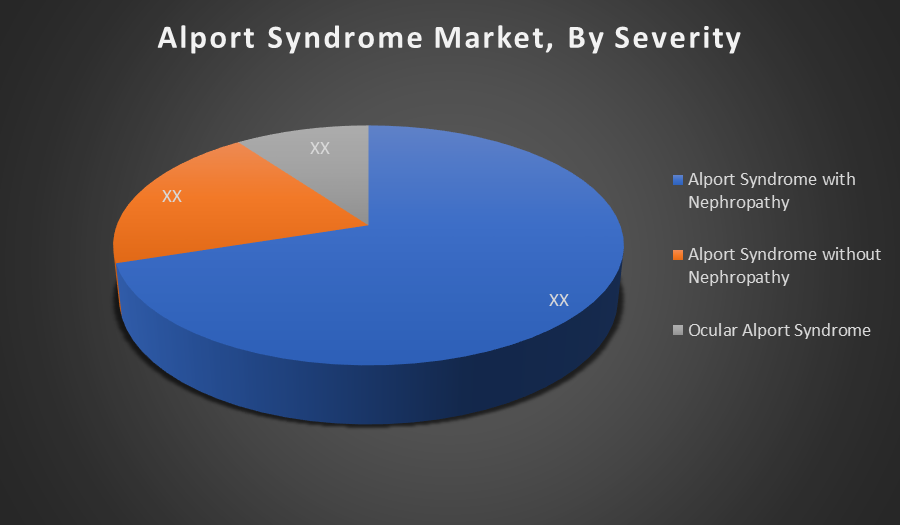
The Alport Syndrome with Nephropathy holds the largest share due to the serious kidney damage it causes, leading to progressive renal failure. This severity increases the need for medical intervention, driving higher demand for diagnosis and treatment options.
Regional Segment Analysis of the Alport Syndrome Market
North America holds the largest share in the Alport Syndrome market due to advanced healthcare infrastructure, high awareness of rare genetic diseases, and widespread availability of advanced diagnostic tools. Strong government support, presence of key market players, and significant investment in research and development further drive market growth. Additionally, favorable reimbursement policies and increasing patient diagnosis rates contribute to the dominance of this region.
The Asia-Pacific region is the fastest-growing market for Alport Syndrome due to rising healthcare expenditures, improving genetic testing facilities, and growing awareness of rare diseases. Increasing adoption of innovative therapies, expanding patient pool, and supportive government initiatives in countries like China and India fuel rapid market expansion. Furthermore, emerging biopharmaceutical companies and collaborations are accelerating the development and accessibility of treatments in this region.
Alport Syndrome Market Key Companies
- AstraZeneca PLC
- Boehringer Ingelheim International GmbH
- Calliditas Therapeutics AB
- CENTOGENE N.V.
- Chinook Therapeutics Inc.
- Daiichi Sankyo Company Limited
- Eloxx Pharmaceuticals Inc.
- Eurofins Scientific SE
- GlaxoSmithKline PLC
- Illumina Inc.
- Invitae Corp.
- Merck & Co. Inc.
- Mylan N.V.
- Natera Inc.
- Others
Alport Syndrome Therapeutics Market Report Scope
- The Alport Syndrome therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Alport Syndrome’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Alport Syndrome therapies is provided, including an evaluation of new treatments expected to influence the current Alport Syndrome treatment market landscape.
- The report includes a detailed review of the Alport Syndrome therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient-Based Alport Syndrome Market Forecasting report offers valuable insights into trends shaping the global Alport Syndrome market, helping to develop effective business strategies.
Alport Syndrome Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Alport Syndrome Therapeutic Approaches in Alport Syndrome
- Review Of Drugs in Development for Alport Syndrome
- Market, Growth, and Trends in Alport Syndrome
- Market Opportunities in Alport Syndrome Treatment
- Effects Of Future Therapies on Alport Syndrome Treatment.
Alport Syndrome Treatment Market Report Key Strengths
- 15 Years Alport Syndrome Market Forecast
- Global Coverage
- Alport Syndrome Epidemiology Segmentation
- Key Cross Competition
Alport Syndrome Treatment Market Report Assessment
- Present Practices in the Alport Syndrome Treatment Market
- Review of Investigational Alport Syndrome Drugs
- Attractiveness of the Alport Syndrome Drug Market
- Alport Syndrome Market Drivers
- Alport Syndrome Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Alport Syndrome market based on the below-mentioned segments:
Global Alport Syndrome Market, By Genetic Mutations
- COL4A3 Mutations
- COL4A4 Mutations
- COL4A5 Mutations
Global Alport Syndrome Market, By Severity
- Alport Syndrome with Nephropathy
- Alport Syndrome without Nephropathy
- Ocular Alport Syndrome
Global Alport Syndrome Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa






