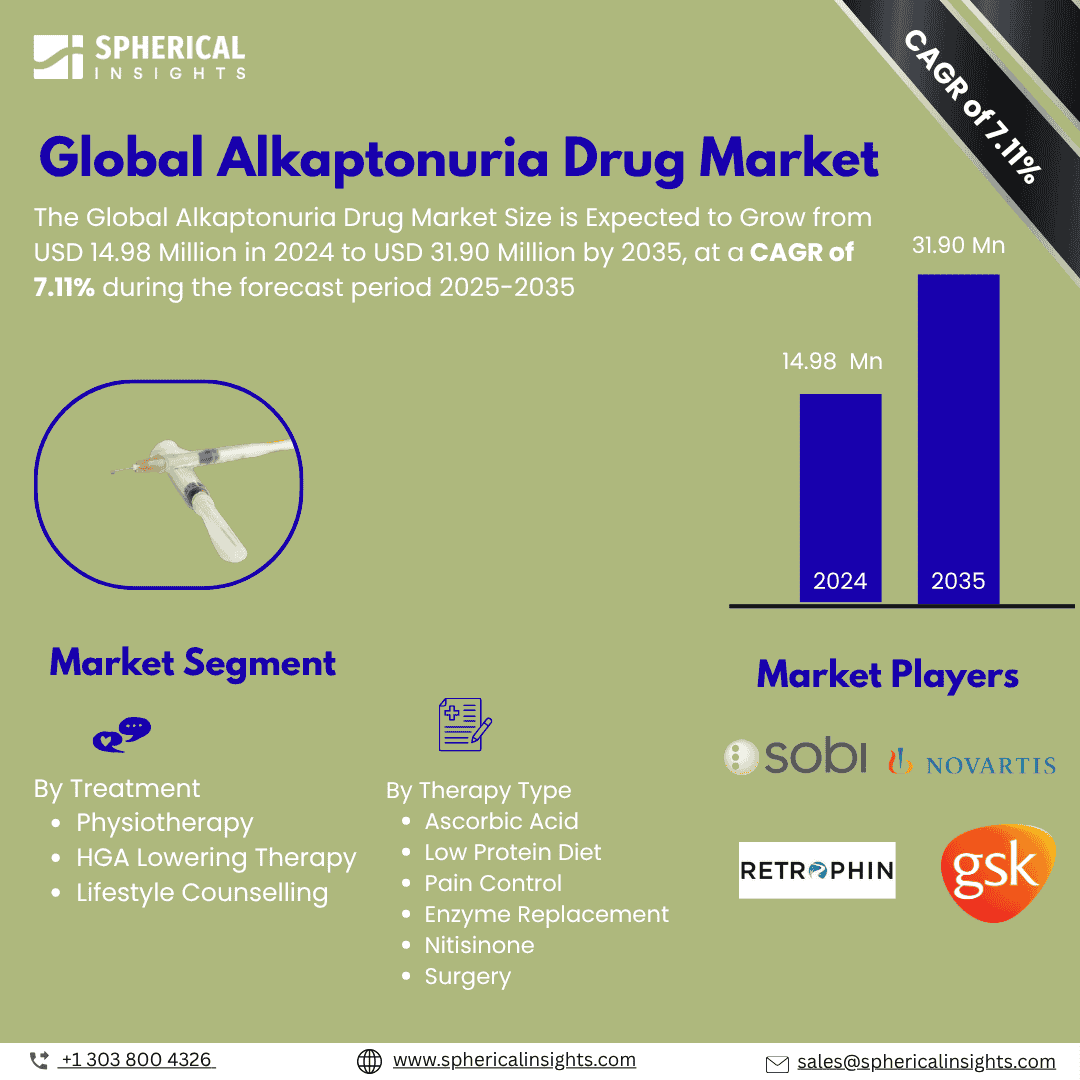
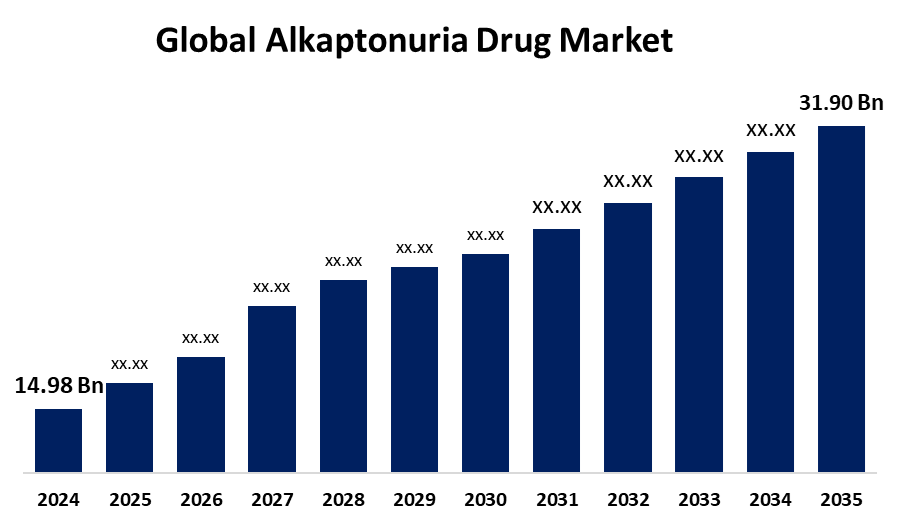
- As per Spherical Insights & Consulting, the Global Alkaptonuria Drug Market is expected to grow from USD 14.98 Million in 2024 to USD 31.90 Million by 2035, at a CAGR of 7.11% during the forecast period 2025-2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Alkaptonuria Drug Market Companies such as Swedish Orphan Biovitrum, SOBI, Retrophin Inc., Novartis AG, Biogen Inc., Pfizer Inc., GlaxoSmithKline plc, Teva Pharmaceuticals, Recordati Rare Diseases, Acer Therapeutics Inc., Amgen Inc., Ultragenyx Pharmaceutical Inc., Sanofi Genzyme, Hoffmann-La Roche Ltd, Bristol-Myers Squibb, and Others.
Alkaptonuria Treatment Market: Understanding and Treatment Algorithm:
Alkaptonuria is a rare inherited metabolic disorder caused by a deficiency of the enzyme homogentisate 1,2-dioxygenase. This leads to the accumulation of homogentisic acid in the body, resulting in darkened urine, joint damage, and tissue pigmentation (ochronosis). Symptoms typically worsen with age, and lifelong monitoring and treatment are required.
Alkaptonuria Diagnosis:
Diagnosis of Alkaptonuria involves urine tests to detect elevated levels of homogentisic acid, which often causes the urine to darken on standing. Confirmatory tests include genetic testing for mutations in the HGD gene. Additional imaging and clinical evaluations help assess joint and organ involvement associated with disease progression.
Alkaptonuria Treatment:
Treatment of Alkaptonuria focuses on managing symptoms and slowing disease progression. It includes the use of nitisinone to reduce homogentisic acid levels, pain control, physiotherapy, and joint replacement surgeries if needed. A low-protein diet and vitamin C supplementation may also help, but lifelong monitoring and supportive care remain essential.
Alkaptonuria Drug Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Alkaptonuria Drug, Gender-specific Diagnosed Incidence of Alkaptonuria Drug, Type-specific Diagnosed Incidence of Alkaptonuria Drug, Age-specific Diagnosed Incidence of Alkaptonuria Drug, Diagnosed Incident Population based on Primary Site of Alkaptonuria Drug, and Diagnosed Incident Population based on Histologic Classification of Alkaptonuria Drug Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of alkaptonuria drug epidemiology in major markets worldwide.
This section offers a global overview of alkaptonuria drug epidemiology in major markets worldwide.
Country Wise- Alkaptonuria Drug Multiforme Epidemiology
- The epidemiology segment provides Alkaptonuria Drug prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
Alkaptonuria Drug Recent Developments:
- In June 2025, Cycle Pharmaceuticals announced that it secured FDA approval for Harliku (nitisinone), the first oral therapy for adult alkaptonuria patients. The decision followed a three-year clinical trial that demonstrated over 95% reduction in urinary homogentisic acid, along with significant improvements in pain, energy, and physical function compared to untreated controls.
Alkaptonuria Drug Marketed Drugs:
- Harliku: Cycle Pharmaceuticals
Harliku (nitisinone) is an oral enzyme inhibitor that reduces the buildup of homogentisic acid, the toxic compound responsible for tissue damage in Alkaptonuria. It is FDA-approved for adults with confirmed Alkaptonuria and has shown significant clinical benefits in reducing ochronosis and improving joint mobility and energy levels.
- Orfadin: Swedish Orphan Biovitrum (SOBI)
Orfadin (nitisinone) is an approved treatment in the EU and UK for Alkaptonuria. Originally developed for hereditary tyrosinemia type 1, it inhibits the same metabolic pathway in AKU, helping to prevent disease progression by lowering homogentisic acid levels. It is part of the DevelopAKUre program in Europe.
- Nityr: Cycle Pharmaceuticals
Nityr (nitisinone) is a tablet formulation bioequivalent to Orfadin, originally approved for hereditary tyrosinemia type 1. It has been considered for off-label use in Alkaptonuria due to its similar mechanism, blocking the breakdown of tyrosine to prevent homogentisic acid accumulation. It offers improved stability and patient compliance over capsule forms.
Alkaptonuria Drug: Emerging Therapies
- HTX-001: It is a small-molecule therapy in preclinical development for Alkaptonuria. It targets metabolic pathways involved in homogentisic acid production to reduce its accumulation. HTX-001 aims to slow or prevent the progression of tissue damage, offering a potential alternative to enzyme-inhibiting therapies like nitisinone.
- Ultragenyx: It is exploring early-stage gene therapy approaches to correct the HGD gene mutation responsible for Alkaptonuria. These therapies aim to restore functional enzyme activity, directly addressing the root cause of the disease and offering the potential for long-term or curative treatment.
- Moderna: It is investigating mRNA technology for rare metabolic disorders, including Alkaptonuria. The therapy would deliver synthetic mRNA encoding the functional HGD enzyme, enabling temporary enzyme expression in target tissues. Though still in exploratory stages, it represents a promising future modality for metabolic correction.
- ACR-001: It is an advanced formulation of sodium phenylbutyrate being evaluated for Alkaptonuria. It works by promoting alternative metabolic pathways that may help reduce the accumulation of homogentisic acid. While originally studied for urea cycle disorders, it is being repurposed in rare metabolic diseases, including AKU.
Alkaptonuria Drug Market Outlook
- The Alkaptonuria drug market focuses on therapies that treat or manage Alkaptonuria, a rare genetic metabolic disorder. It includes enzyme inhibitors like nitisinone, supportive treatments, dietary management products, and emerging gene therapies, aimed at reducing homogentisic acid accumulation and preventing disease progression.
- Growing awareness of rare diseases, availability of newborn screening programs, increasing research in metabolic disorders, and the FDA’s support for orphan drugs are key market drivers. Rising adoption of nitisinone and improved access to diagnosis and treatment also significantly propel market growth.
- Opportunities exist in developing gene therapies and novel enzyme replacements targeting the HGD mutation. Expanding healthcare access in emerging markets and strategic collaborations between biotech firms and research organizations could accelerate innovation and reach for Alkaptonuria treatment worldwide, especially where current therapies are limited.
- Government support includes orphan drug designations, research grants, newborn screening mandates, and regulatory incentives encouraging development for ultra-rare disorders. These policies help pharmaceutical firms invest in therapies for Alkaptonuria, ensuring faster clinical trial approvals and broader global treatment access.
- Low patient population limits commercial incentives and investment in new therapies.
- The market is expected to grow steadily due to the increasing adoption of nitisinone and advancement in gene-based treatment approaches.
Alkaptonuria Drug Market Segmentation
By Therapy Type:
- Physiotherapy
- HGA Lowering Therapy
- Lifestyle Counselling
HGA-Lowering Therapy led the global Alkaptonuria drug market due to its direct focus on reducing homogentisic acid accumulation, the key driver of disease progression. It became the most adopted approach because it addresses root causes and is supported by regulatory approvals and clinical evidence demonstrating slowed ochronosis and symptom relief.
By Treatment Type:
- Ascorbic Acid
- Low Protein Diet
- Pain Control
- Enzyme Replacement
- Nitisinone
- Surgery
Nitisinone dominated within the treatment type segment, accounting for the largest share. It acts as an effective enzyme inhibitor that significantly reduces homogentisic acid levels, slows disease progression, and improves patient outcomes. Its regulatory approvals and growing clinical acceptance cemented its leading position among available therapies.
Regional Segment Analysis of the Alkaptonuria Drug Market
North America dominated the Alkaptonuria drug market by leveraging its advanced healthcare infrastructure, strong regulatory support, and substantial rare disease research funding. Robust genetic screening and specialized metabolic clinics in the U.S. and Canada facilitate early diagnosis and effective treatment. Combined with heightened awareness and reimbursement models, this region maintained its market leadership through comprehensive patient care pathways.
The Asia-Pacific region exhibited the fastest growth in the alkaptonuria market, driven by expanding genetic screening programs, rising healthcare investment, and increased rare disease awareness in countries line China and India. Partnerships between international pharmaceutical firms and local health systems improved diagnostic access and therapy availability. Moreover, government support for orphan disease initiatives and better healthcare infrastructure accelerated treatment adoption across the region.
Alkaptonuria Drug Market Key Companies
- Swedish Orphan Biovitrum
- SOBI
- Retrophin Inc.
- Novartis AG
- Biogen Inc.
- Pfizer Inc.
- GlaxoSmithKline plc
- Teva Pharmaceuticals
- Recordati Rare Diseases
- Acer Therapeutics Inc.
- Amgen Inc.
- Ultragenyx Pharmaceutical Inc.
- Sanofi Genzyme
- Hoffmann-La Roche Ltd
- Bristol-Myers Squibb
- Others
Alkaptonuria Drug Therapeutics Market Report Scope
- The Alkaptonuria Drug Therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Alkaptonuria Drug’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Alkaptonuria Drug therapies is provided, including an evaluation of new treatments expected to influence the current Alkaptonuria Drug treatment market landscape.
- The report includes a detailed review of the Alkaptonuria Drug therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient-Based Alkaptonuria Drug Market Forecasting report offers valuable insights into trends shaping the global Alkaptonuria Drug market, helping to develop effective business strategies.
Alkaptonuria Drug Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Alkaptonuria Drug Therapeutic Approaches in Alkaptonuria Drug
- Review Of Drugs in Development for Alkaptonuria Drug
- Market, Growth, and Trends in Alkaptonuria Drug
- Market Opportunities in Alkaptonuria Drug Treatment
- Effects Of Future Therapies on Alkaptonuria Drug Treatment.
Alkaptonuria Drug Treatment Market Report Key Strengths
- 15 Years Alkaptonuria Drug Market Forecast
- Global Coverage
- Alkaptonuria Drug Epidemiology Segmentation
- Key Cross Competition
Alkaptonuria Drug Treatment Market Report Assessment
- Present Practices in the Alkaptonuria Drug Treatment Market
- Review of Investigational Alkaptonuria Drug Drugs
- Attractiveness of the Alkaptonuria Drug Drug Market
- Alkaptonuria Drug Market Drivers
- Alkaptonuria Drug Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the alkaptonuria drug market based on the below-mentioned segments:
Global Alkaptonuria Drug Market, By Therapy Type
- Physiotherapy
- HGA Lowering Therapy
- Lifestyle Counselling
Global Alkaptonuria Drug Market, By Treatment Type
- Ascorbic Acid
- Low Protein Diet
- Pain Control
- Enzyme Replacement
- Nitisinone
- Surgery
Global Alkaptonuria Drug Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa