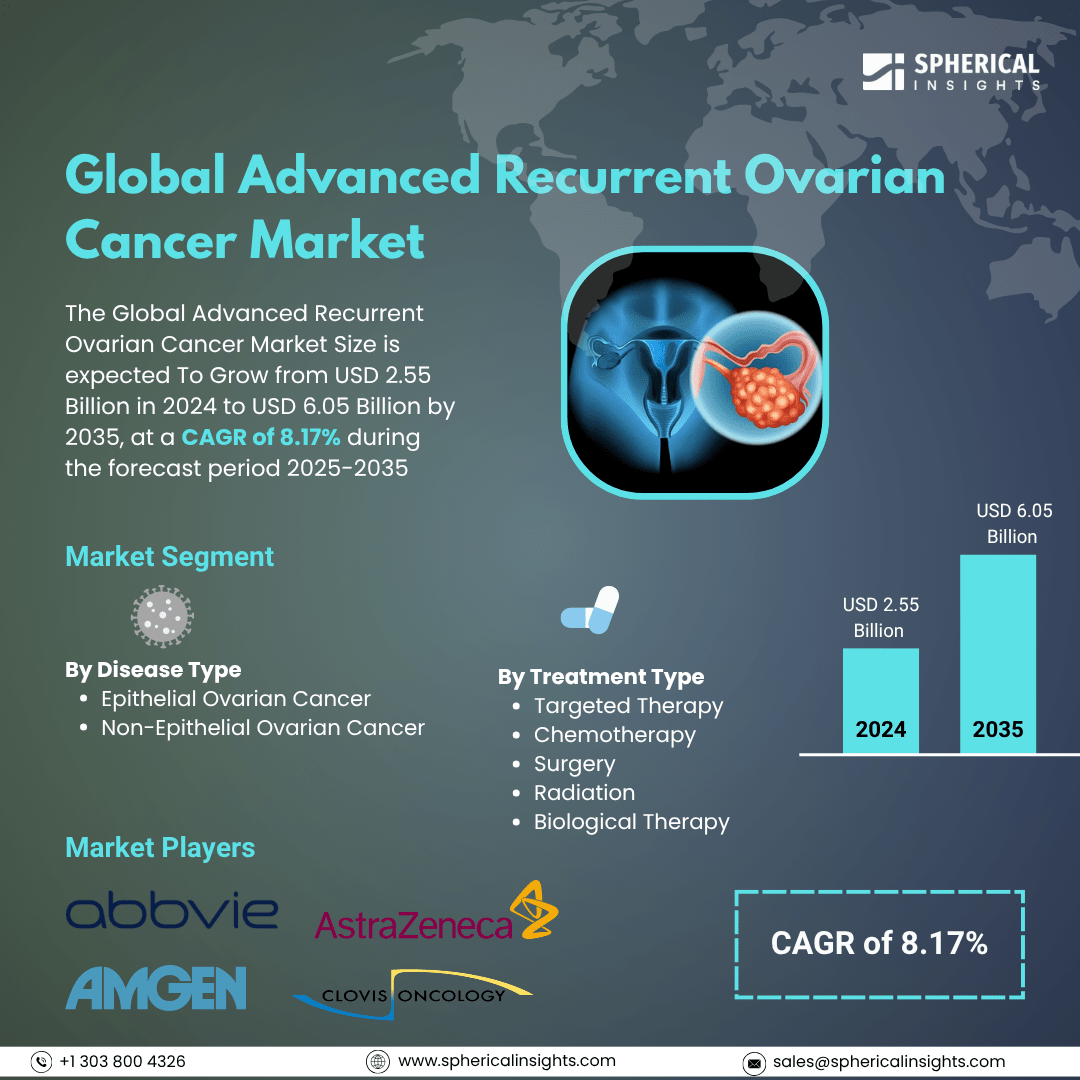
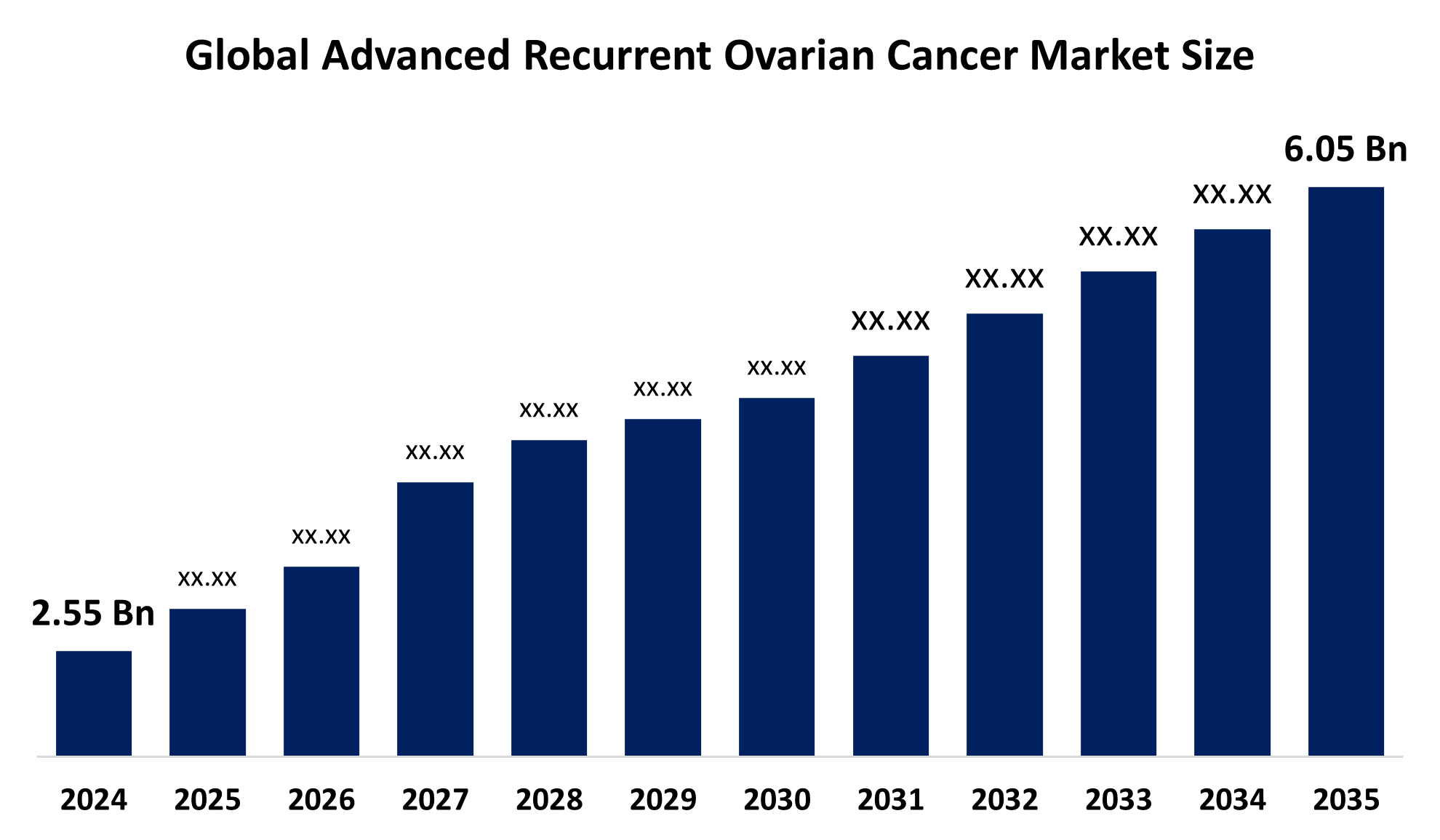
- As per Spherical Insights & Consulting, The Global Advanced Recurrent Ovarian Cancer Market Size is expected To row from USD 2.55 Billion in 2024 to USD 6.05 Billion by 2035, at a CAGR of 8.17% during the forecast period 2025-2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- Key players in the advanced recurrent ovarian cancer market include prominent companies such as AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Clovis Oncology, Eli Lilly, Exelixis, Genentech, GSK, ImmunoGen, Johnson & Johnson, Merck & Co., Novartis, Pfizer, Roche, among others.
Advanced Recurrent Ovarian Cancer Treatment Market: Understanding and Treatment Algorithm:
Advanced recurrent ovarian cancer refers to ovarian cancer that has returned after initial treatment and has spread beyond the ovaries. It is often more difficult to treat due to resistance to prior therapies. Management typically involves chemotherapy, targeted therapies, or clinical trials aimed at prolonging survival and improving quality of life.
Advanced Recurrent Ovarian Cancer Diagnosis:
Diagnosis of advanced recurrent ovarian cancer involves a combination of clinical evaluation, imaging tests (such as CT or PET scans), and tumor marker assessments like CA-125 levels. Symptoms prompting evaluation may include abdominal pain, bloating, or changes in bowel habits. Biopsy or surgical confirmation may be required to distinguish recurrence from other conditions.
Advanced Recurrent Ovarian Cancer Treatment
Treatment of advanced recurrent ovarian cancer depends on prior therapy, treatment-free interval, and tumor biology. Options include platinum-based or non-platinum chemotherapy, targeted therapies like PARP inhibitors or anti-angiogenic agents, and antibody–drug conjugates. Clinical trials are often considered. The goal is to control disease, manage symptoms, and improve or maintain quality of life.
Advanced Recurrent Ovarian Cancer Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Advanced Recurrent Ovarian Cancer, Gender-specific Diagnosed Incidence of Advanced Recurrent Ovarian Cancer, Type-specific Diagnosed Incidence of Advanced Recurrent Ovarian Cancer, Age-specific Diagnosed Incidence of Advanced Recurrent Ovarian Cancer, Diagnosed Incident Population based on Primary Site of Advanced Recurrent Ovarian Cancer, and Diagnosed Incident Population based on Histologic Classification of Advanced Recurrent Ovarian Cancer Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of advanced recurrent ovarian cancer epidemiology in major markets worldwide.
Country Wise- Advanced Recurrent Ovarian Cancer Multiforme Epidemiology
- The epidemiology segment provides Advanced Recurrent Ovarian Cancer prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
Advanced Recurrent Ovarian Cancer Market Recent Developments:
- In December 2024, GSK announced that its late-stage FIRST-ENGOT-OV44 trial met the primary endpoint, as the combination of Jemperli with chemotherapy and Zejula significantly improved progression-free survival in first-line advanced ovarian cancer. However, the key secondary endpoint of overall survival was not statistically significant.
Advanced Recurrent Ovarian Cancer Marketed Drugs:
Keytruda (pembrolizumab) is an anti-PD-1 monoclonal antibody that enhances the immune response against tumor cells. It is FDA-approved for recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) and used in both first-line and previously treated patients based on PD-L1 expression. In ovarian cancer, it is often used in clinical trials or off-label in combination regimens but does not have standalone FDA approval for this indication yet.
Avastin (bevacizumab) is a VEGF inhibitor that blocks angiogenesis, thereby limiting tumor blood supply. It is FDA-approved for use in combination with chemotherapy for platinum-resistant recurrent ovarian cancer and also approved in frontline and maintenance therapy settings.
Elahere (mirvetuximab soravtansine) is an antibody-drug conjugate targeting folate receptor alpha (FRα), delivering cytotoxic agents directly to cancer cells. It is FDA-approved for platinum-resistant ovarian cancer patients with high FR expression.
Advanced Recurrent Ovarian Cancer: Emerging Therapies
- BNT327: It is a bispecific antibody in late-stage trials for Advanced Recurrent Ovarian Cancer. It targets two immune checkpoints to enhance T-cell activation and tumor response, aiming to improve outcomes in patients resistant to standard PD-1/PD-L1 therapies.
- Ubamatamab: It is a bispecific antibody targeting MUC16 on ovarian cancer cells and CD3 on T-cells, designed to engage T-cells directly to kill tumor cells. It is in early-phase clinical trials showing promising activity in platinum-resistant ovarian cancer.
- Elahere: It is an antibody-drug conjugate targeting folate receptor alpha (FRα), delivering cytotoxic payloads to tumor cells. It is FDA-approved for platinum-resistant ovarian cancer with high FRα expression and is being investigated in combination therapies.
- Olvi-Vec: It is an oncolytic viral therapy designed to selectively infect and kill ovarian cancer cells while stimulating an immune response. It is currently in clinical trials for recurrent ovarian cancer.
Advanced Recurrent Ovarian Cancer Market Outlook
- The advanced recurrent ovarian cancer market encompasses therapies and treatments targeting ovarian cancer that has returned after initial therapy. It includes chemotherapies, targeted agents, immunotherapies, and emerging bispecific antibodies aimed at improving survival and quality of life in resistant or late-stage patients.
- Market growth is driven by rising ovarian cancer incidence, unmet needs in resistant recurrent cases, advances in targeted and immunotherapies, increasing adoption of personalized medicine, regulatory approvals of novel agents, and growing awareness among patients and healthcare providers about innovative treatment options improving outcomes.
- Significant opportunities exist in developing bispecific antibodies, antibody-drug conjugates, combination immunotherapies, and targeted therapies for platinum-resistant ovarian cancer. Expansion into earlier treatment lines, biomarker-driven personalized approaches, and emerging markets also offer growth potential amid evolving clinical trial successes and unmet patient needs.
- Governments support ovarian cancer control through funding, early detection, and access to advanced treatments.
- Limited treatment options and high resistance rates in advanced recurrent ovarian cancer pose significant challenges to improving patient outcomes.
- Market growth is driven by rising ovarian cancer cases, unmet treatment needs, and advances in targeted and immunotherapies.
Advanced Recurrent Ovarian Cancer Market Segmentation
By Disease Type:
- Epithelial Ovarian Cancer
- Non-Epithelial Ovarian Cancer
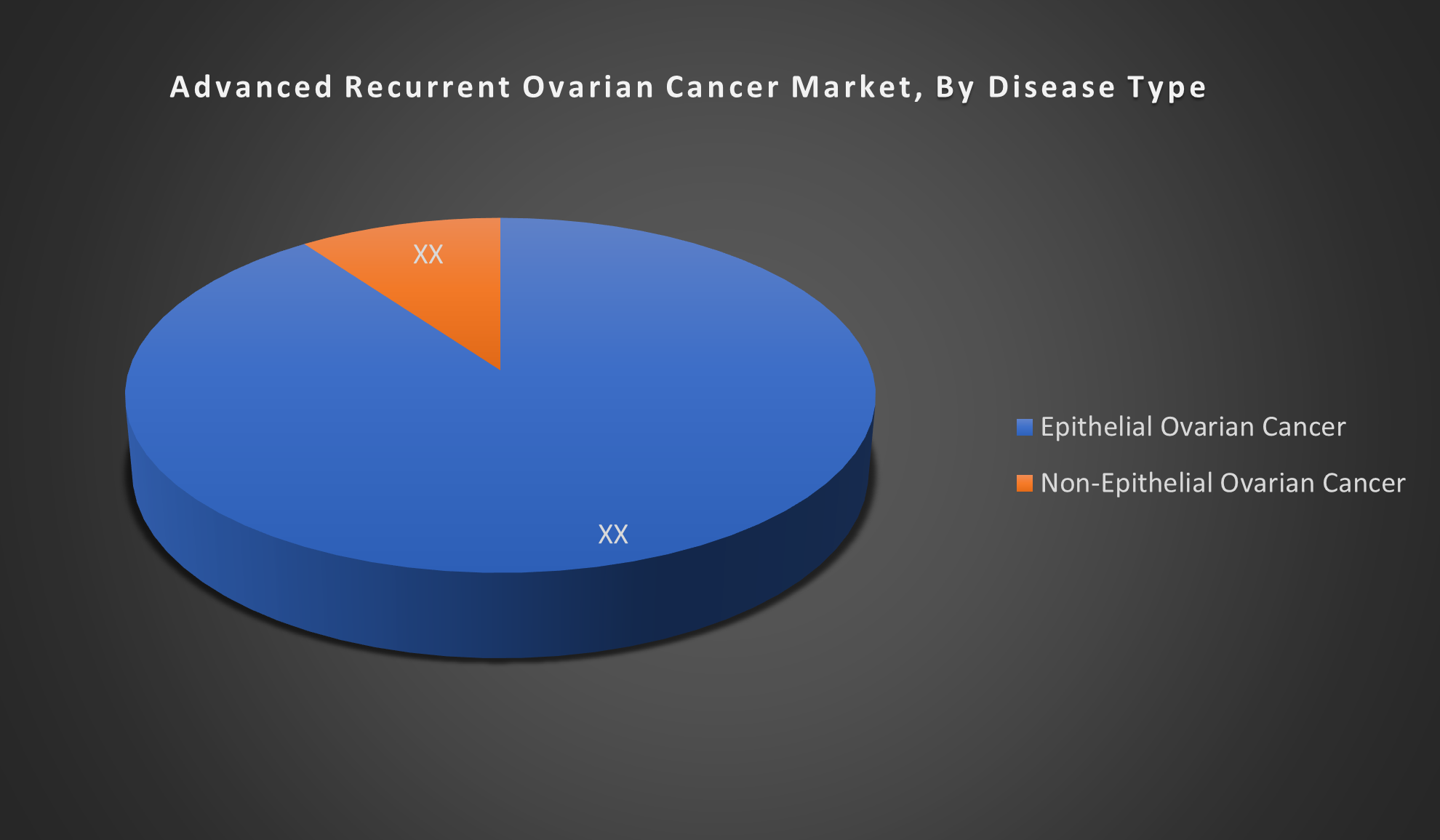
The Epithelial Ovarian Cancer (EOC) segment is dominant due to its higher prevalence, accounting for about 90% of ovarian cancer cases. Its aggressive nature and frequent recurrence drive greater demand for advanced therapies and clinical research focus.
By Treatment Type:
- Targeted Therapy
- Chemotherapy
- Surgery
- Radiation
- Biological Therapy
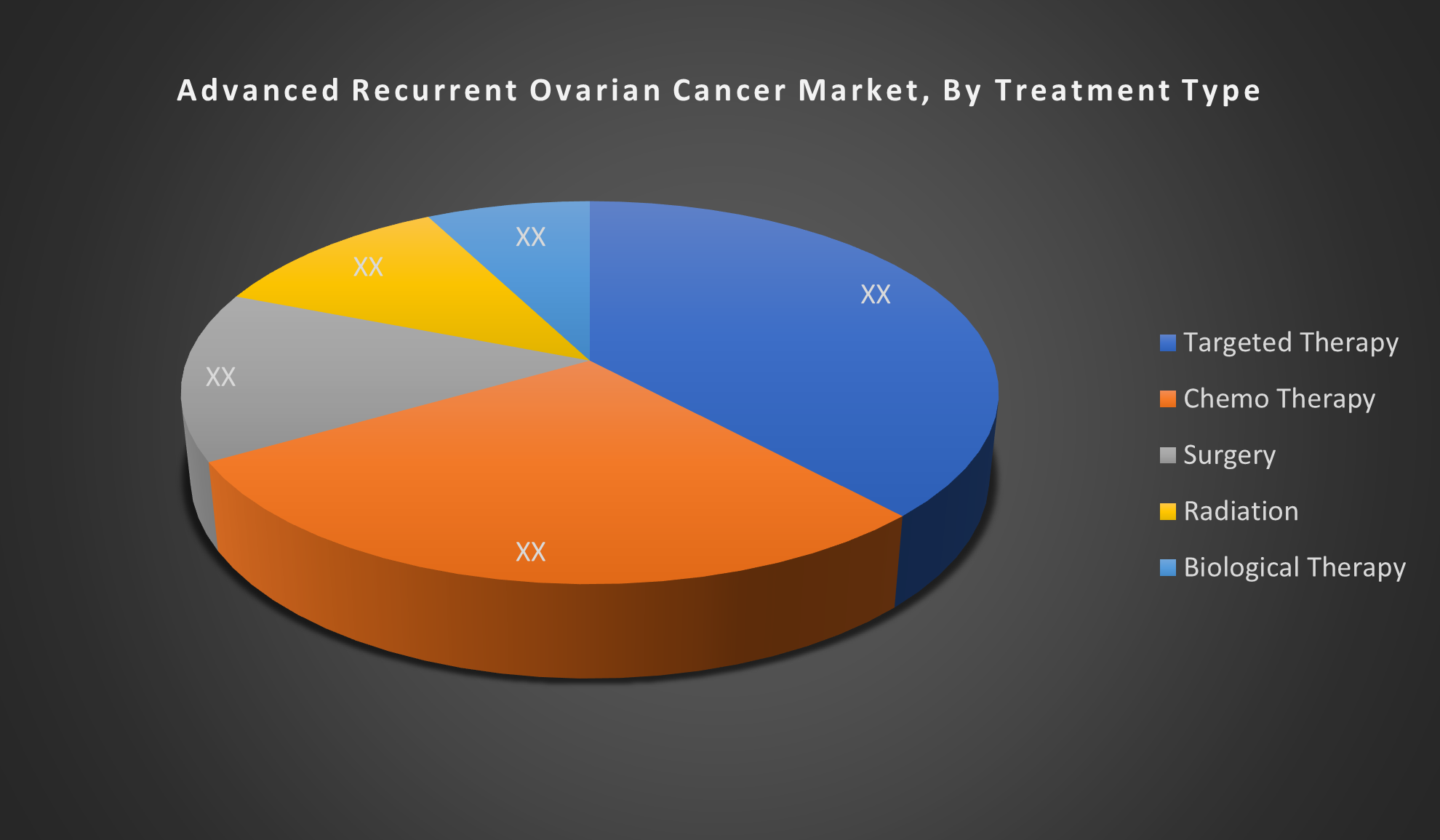
The Targeted Therapy segment is dominated due to its improved efficacy and safety over traditional treatments. Advances in PARP inhibitors, immunotherapies, and antibody-drug conjugates offer personalized, effective options, driving higher adoption compared to chemotherapy, surgery, or radiation in recurrent ovarian cancer.
Regional Segment Analysis of the Advanced Recurrent Ovarian Cancer Market
North America dominates the Global Advanced Recurrent Ovarian Cancer Market owing to its well-established healthcare infrastructure, high patient awareness, and significant investment in research and development. The region benefits from early cancer detection programs and the widespread adoption of advanced therapies, including immunotherapy, targeted treatments, and PARP inhibitors. Additionally, supportive reimbursement policies and a strong presence of key pharmaceutical players further drive market growth. Lifestyle risk factors such as tobacco and alcohol use also contribute to a larger patient population, increasing treatment demand.
The Asia-Pacific region is the fastest-growing market, fueled by increasing ovarian cancer incidence rates in populous countries like China and India. Rapid improvements in healthcare infrastructure, rising disposable incomes, and government initiatives to enhance cancer awareness and screening are accelerating market expansion. Although challenges like limited access to advanced treatments and uneven healthcare quality persist, growing collaborations between local and global pharma companies and increasing clinical trials are expected to bolster growth in this region over the coming years.
Advanced Recurrent Ovarian Cancer Market Key Companies
Advanced Recurrent Ovarian Cancer Therapeutics Market Report Scope
- The Advanced Recurrent Ovarian Cancer therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Advanced Recurrent Ovarian Cancer’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Advanced Recurrent Ovarian Cancer therapies is provided, including an evaluation of new treatments expected to influence the current Advanced Recurrent Ovarian Cancer treatment market landscape.
- The report includes a detailed review of the Advanced Recurrent Ovarian Cancer therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient-Based Advanced Recurrent Ovarian Cancer Market Forecasting report offers valuable insights into trends shaping the global Advanced Recurrent Ovarian Cancer market, helping to develop effective business strategies.
Advanced Recurrent Ovarian Cancer Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Advanced Recurrent Ovarian Cancer Therapeutic Approaches in Advanced Recurrent Ovarian Cancer
- Review Of Drugs in Development for Advanced Recurrent Ovarian Cancer
- Market, Growth, and Trends in Advanced Recurrent Ovarian Cancer
- Market Opportunities in Advanced Recurrent Ovarian Cancer Treatment
- Effects Of Future Therapies on Advanced Recurrent Ovarian Cancer Treatment.
Advanced Recurrent Ovarian Cancer Treatment Market Report Key Strengths
- 15 Years Advanced Recurrent Ovarian Cancer Market Forecast
- Global Coverage
- Advanced Recurrent Ovarian Cancer Epidemiology Segmentation
- Key Cross Competition
Advanced Recurrent Ovarian Cancer Treatment Market Report Assessment
- Present Practices in the Advanced Recurrent Ovarian Cancer Treatment Market
- Review of Investigational Advanced Recurrent Ovarian Cancer Drugs
- Attractiveness of the Advanced Recurrent Ovarian Cancer Drug Market
- Advanced Recurrent Ovarian Cancer Market Drivers
- Advanced Recurrent Ovarian Cancer Market Barriers
- SWOT
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the advanced recurrent ovarian cancer market based on the below-mentioned segments:
Global Advanced Recurrent Ovarian Cancer Market, By Disease Type
- Epithelial Ovarian Cancer
- Non-Epithelial Ovarian Cancer
Global Advanced Recurrent Ovarian Cancer Market, By Treatment Type
- Targeted Therapy
- Chemotherapy
- Surgery
- Radiation
- Biological Therapy
Global Advanced Recurrent Ovarian Cancer Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa