Global Virtual Clinical Trials Market Size, Share, and COVID-19 Impact Analysis, By Study Design (Interventional, Observational, and Expanded Access), By Indication (Oncology and Cardiovascular Disease), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033.
Industry: HealthcareGlobal Virtual Clinical Trials Market Insights Forecasts to 2033
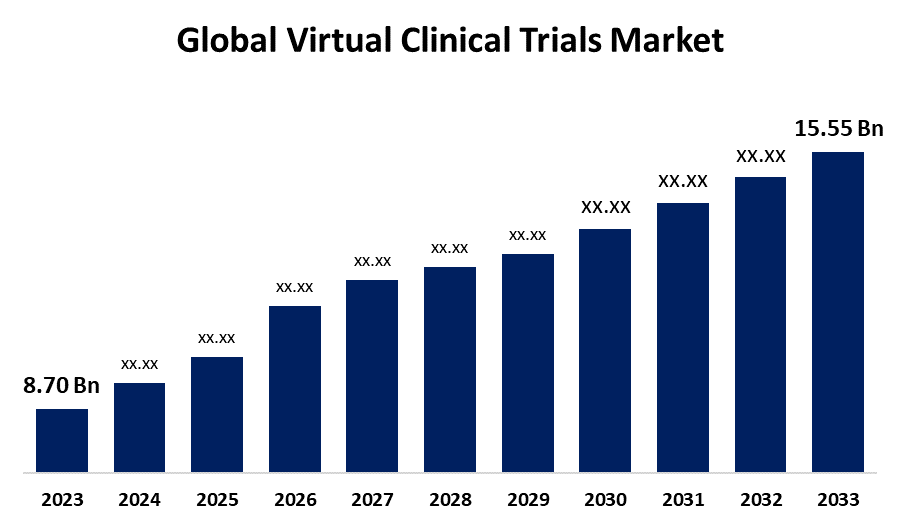
- The Global Virtual Clinical Trials Market Size was estimated at USD 8.70 Billion in 2023
- The Market Size is Expected to Grow at a CAGR of around 5.98% from 2023 to 2033
- The Worldwide Virtual Clinical Trials Market Size is Expected to Reach USD 15.55 Billion by 2033
- Asia Pacific is expected to grow the fastest during the forecast period.
Get more details on this report -
The Global Virtual Clinical Trials Market size was worth around USD 8.70 Billion in 2023 and is predicted to Grow to around USD 15.55 Billion By 2033 with a compound annual growth rate (CAGR) of 5.98% between 2023 and 2033. Virtual clinical trials are becoming more popular because of patient recruitment and retention, precision of data, reduced costs, and enhanced efficiency. Moreover, virtual trials facilitate the collection of real-world data in real-world settings and are available to a wide range of patient populations. Government initiatives, advances in technology, and increased demand for decentralized and patient-friendly trial methods are expected to drive the growth of the virtual clinical trials market during the next several years.
Market Overview
Global virtual clinical trials market refers to the use of digital technologies to remotely carry out clinical trials through means such as telemedicine, mobile apps, and remote monitoring devices. Additionally, the virtual clinical trials market is changing fast as healthcare professionals and researchers turn to digital solutions that simplify clinical research. Virtual trials, or decentralized clinical trials (DCTs), use digital health technologies to enable patients to participate remotely, eliminating the need for conventional trial sites. This not only makes clinical research more convenient but also maintains continuity, especially in studies of chronic and rare diseases that conventionally experience logistical difficulties. Major digital advancements like AI-based recruitment, data analytics, and telemedicine are assisting in maximizing trial efficiency and transparency, allowing pharmaceutical companies to respond quickly to patient needs. Moreover, sophisticated data management solutions are enhancing data quality and regulatory compliance, further solidifying virtual trials as a preferred approach for patients and providers.
Report Coverage
This research report categorizes the global virtual clinical trials market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global virtual clinical trials market. Recent market developments and competitive strategies such as expansion, type launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global virtual clinical trials market.
Global Virtual Clinical Trials Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 8.70 Billion |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 5.98% |
| 2033 Value Projection: | USD 15.55 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 190 |
| Tables, Charts & Figures: | 96 |
| Segments covered: | By Study Design, By Indication, By Region and COVID-19 Impact Analysis |
| Companies covered:: | ICON, plc, Parexel International Corporation, IQVIA, Covance, PRA Health Sciences, LEO Innovation Lab, Medidata, Oracle, CRF Health, Clinical Ink, Medable, Inc., Halo Health Systems, Croprime, and other key vendors. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
This market is very much concentrated on the needs of the patients that assist the market to develop at a very fast pace. It provides convenience to the patients in monitoring their health status which makes a large number of patients go for such devices. It minimizes the necessity of going from one location to another such development drives the growth of the market. It assists patients residing in any part of the world and is highly travel-friendly. With the advancement in technology, it is becoming simple for patients to monitor heart rate, blood pressure, oxygen level and many more that can be tested without any assistance from professionals.
Restraining Factors
One of the main constraints related to this industry is regulatory uncertainties. There is a difference in the regulatorily norms between regions for the acceptance and conductance of virtual mode of clinical trials. Moreover, data security and the possibility of digital guidance among patients are of serious concern. These intricacies all together are responsible for the continuous challenges in extensive implementation of decentralized clinical trials.
Market Segmentation
The global virtual clinical trials market share is classified into study design and indication.
- The interventional segment held the largest share in 2023 and is expected to grow at a significant CAGR during the forecast period.
Based on the study design, the virtual clinical trials market is categorized into interventional, observational, and expanded access. Among these, the interventional segment held the largest share in 2023 and is expected to grow at a significant CAGR during the forecast period. This growth is attributable to increasing demand for minimally invasive surgeries, advances in surgical instruments, and rising incidence of chronic diseases. Enhanced patient outcomes, shorter recovery periods, and rising healthcare expenditures worldwide further drive the growth of the segment and expected strong CAGR over the forecast period.
- The oncology segment held the largest share in 2023 and is expected to grow at a significant CAGR during the forecast period.
Based on the indication, the virtual clinical trials market is categorized into oncology and cardiovascular disease. Among these, the oncology segment held the largest share in 2023 and is expected to grow at a significant CAGR during the forecast period. The segment is also expected to drive the largest market share during the forecast period. This is due to the increasing number of cancer cases in the world and the growing oncology clinical trials. Cancer patients are the most susceptible to COVID-19 pandemic. Investigators and sponsors of oncology clinical trials have rapidly adapted virtual and remote trials to safeguard patients and continue trials.
Regional Segment Analysis of the Virtual Clinical Trials Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
North America is anticipated to hold the largest share of the virtual clinical trials market over the predicted timeframe.

Get more details on this report -
North America is anticipated to hold the largest share of the virtual clinical trials market over the predicted timeframe. The growing need for patient-centric strategies and decentralized trial designs, where virtual clinical trials provide answers to improving the level of patient participation and retention rates. Advances in technology, specifically in telemedicine, wearables, and remote monitoring devices, drive the adoption of virtual trial methods forward, allowing continuous data collection and analysis and lowering the workload on both patients and researchers.
Asia Pacific is expected to grow at the fastest CAGR in the virtual clinical trials market during the forecast period. The growth rate of cardiovascular diseases and surge in geriatric population are few among factors likely to drive a growth of a virtual clinical trials market during the forecast period. Higher number of clinical trials aimed at discovering strong and effective remedies for reducing or curing and preventing spread of cardiovascular as well as other diseases will tend to improve the demand for virtual clinical trials throughout the forecast period.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global virtual clinical trials market along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- ICON, plc
- Parexel International Corporation
- IQVIA
- Covance
- PRA Health Sciences
- LEO Innovation Lab
- Medidata
- Oracle
- CRF Health
- Clinical Ink
- Medable, Inc.
- Halo Health Systems
- Croprime
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In July 2023, Signant Health completed the acquisition of DSG, strategically enhancing its eClinical solution suite for traditional as well as decentralized clinical trials. With the integration of DSG's single platform, the acquisition helped create an end-to-end trial ecosystem with sophisticated software, analytics, and logistics solutions to support robust study conduct and data generation across all modalities, thus achieving the vision of complete digitalization of clinical trials.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2023 to 2033. Spherical Insights has segmented the global virtual clinical trials market based on the below-mentioned segments:
Global Virtual Clinical Trials Market, By Study Design
- Interventional
- Observational
- Expanded Access
Global Virtual Clinical Trials Market, By Indication
- Oncology
- Cardiovascular Disease
Global Virtual Clinical Trials Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the global virtual clinical trials market over the forecast period?The global virtual clinical trials market is projected to expand at a CAGR of 5.98 % during the forecast period.
-
2. What is the market size of the global virtual clinical trials market?The global virtual clinical trials market Size is expected to grow from USD 8.70 Billion in 2023 to USD 15.55 Billion by 2033, at a CAGR of 5.98% during the forecast period 2023-2033.
-
3. Which region holds the largest share of the global virtual clinical trials market?North America is anticipated to hold the largest share of the global virtual clinical trials market over the predicted timeframe.
Need help to buy this report?