Global Viral Vectors for Non-human Primates Market Size, Share, and COVID-19 Impact Analysis, By Vector Type (Adenoviral Vectors, Adeno-associated Vectors, Retroviral Vectors, Lentiviral Vectors, and Others), By Type of Non-human Primates (Marmosets, Rhesus Macaques, Cynomolgus Monkey, and Others), By Application (Gene Therapy and Vaccine Research), By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 - 2035
Industry: HealthcareGlobal Viral Vectors for Non-human Primates Market Insights Forecasts to 2035
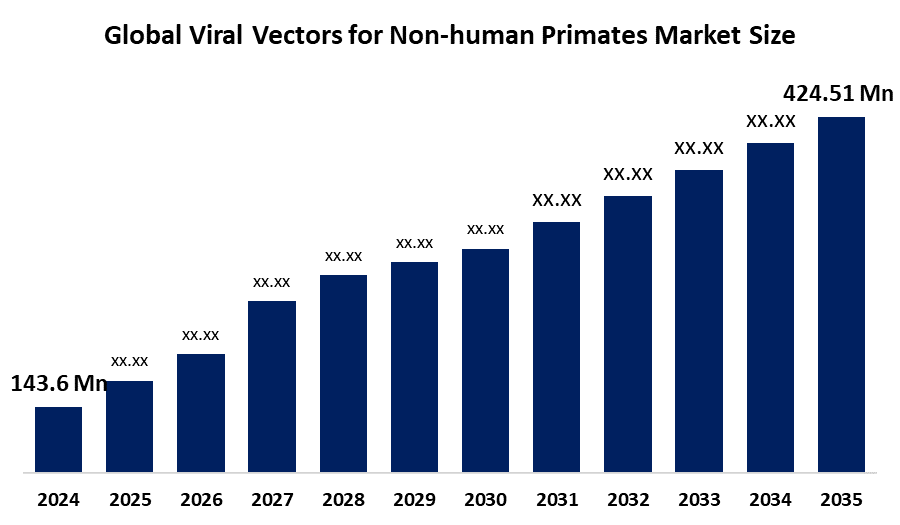
- The Global Viral Vectors for Non-human Primates Market Size Was Estimated at USD 143.6 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 10.36% from 2025 to 2035
- The Worldwide Viral Vectors for Non-human Primates Market Size is Expected to Reach USD 424.51 Million by 2035
- Asia Pacific is expected to grow the fastest during the forecast period.
Get more details on this report -
According to a research report published by Spherical Insights and Consulting, The Global Viral Vectors for Non-human Primates Market Size was worth around USD 143.6 Million in 2024, growing to USD 158.8 Million in 2025, and is predicted to grow to around USD 424.51 Million by 2035 with a compound annual growth rate (CAGR) of 10.36% from 2025 to 2035. The growth is fueled by rising utilization of non-human primates (NHPs) in preclinical gene therapy studies, particularly for diseases that are complex in nature, such as neurological diseases. Technological innovations in viral vector technology provide targeted gene delivery, and increased validation of safety and efficacy in NHP models is necessary before trials on humans.
Global Viral Vectors for Non-human Primates Market Forecast and Revenue Outlook
- 2024 Market Size: USD 143.6 Million
- 2025 Market Size USD 158.8 Million
- 2035 Projected Market Size: USD 424.51 Million
- CAGR (2025-2035): 10.36%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
Market Overview
The viral vector market for non-human primates (NHP) refers to based on the manufacture and utilization of viral vectors, genetically modified viruses intended to deliver genetic material into cells, for research and treatment purposes with non-human primates. Viral vectors are valuable reagents in gene therapy, vaccine production, and cutting-edge biomedical investigation, especially as NHPs are close to human physiology and therefore very useful models for disease study and assessment of new treatment modalities. Expansion is fueled by rising demand for gene therapies, growth in biomedical research activities, and increased use of NHP models for preclinical research.
The prospects are rewarding with advances in genetic engineering and vector technology opening up new paths for the treatment of complicated diseases, and emerging economies becoming more ready to adopt these technologies. Major market participants, such as major pharmaceutical and biotech companies such as Merck KGaA, Lonza Group, Thermo Fisher Scientific, Andelyn Biosciences, Biovian, 4D Molecular Therapeutics, heavily invest in developing new viral vector technologies to enhance delivery efficiency and safety. Recent government initiatives worldwide, such as extra funds for research in gene therapy and fewer regulatory barriers, also drive market development through faster innovation and product development. Researchers from Kyoto University, aided by Japan's MEXT in its WPI program, in March 2025 successfully administered a transgene to cynomolgus monkeys using a non-viral system, enhancing gene delivery techniques and going beyond ethical issues surrounding the utilization of viral vectors.
Key Market Insights
- North America is expected to account for the largest share in the viral vectors for non-human primates market during the forecast period.
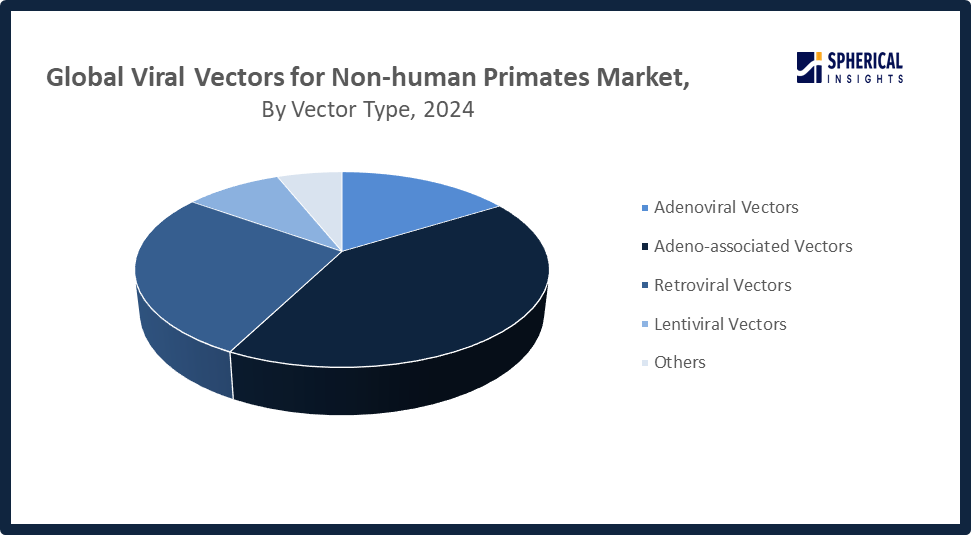
- In terms of vector type, the adeno-associated vectors segment is projected to lead the viral vectors for non-human primates market throughout the forecast period
- In terms of type of non-human primates, the rhesus macaques segment captured the largest portion of the market
- In terms of application, the gene therapy segment captured the largest portion of the market
Viral Vectors for Non-human Primates Market Trends
- Advanced vector engineering improves delivery efficiency and safety.
- Increasing adoption of viral vectors in gene therapy research drives market growth.
- Growing use of non-human primates in preclinical studies enhances market demand.
- Rising focus on personalized medicine boosts vector development.
- Expansion of biotechnology collaborations accelerates innovation.
Report Coverage
This research report categorizes the viral vectors for non-human primates market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyzes the key growth drivers, opportunities, and challenges influencing the viral vectors for non-human primates market. Recent market developments and competitive strategies, such as expansion, type launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyzes their core competencies in each sub-segment of the viral vectors for non-human primates market.
Global Viral Vectors for Non-human Primates Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 143.6 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 10.36% |
| 2035 Value Projection: | USD 424.51 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 245 |
| Tables, Charts & Figures: | 122 |
| Segments covered: | By Vector Type, By Type of Non-human Primates, By Application, By Region |
| Companies covered:: | Lonza Group AG, REGENXBIO Inc., Oxford Biomedica, Merck KGaA, 4D Molecular Therapeutics, Biovian Oy, Thermo Fisher Scientific Inc., CRISPR Therapeutics, Barinthus Biotherapeutics, Genezen, Andelyn Biosciences, Fujifilm Diosynth Biotechnologies, Others |
| Pitfalls & Challenges: | Covid 19 Impact Challenges, Future, Growth and Analysis |
Get more details on this report -
Driving factors
The growing need for gene therapies, sophisticated biomedical research, and enhanced applications of NHP models propel the market for viral vectors. Increased need for gene therapies propels the market for viral vectors because these therapies need effective delivery mechanisms for the treatment of genetic diseases. Sophisticated biomedical research depends more and more on viral vectors to conduct disease research and develop therapies, which enhances growth in the market. Furthermore, the increasing application of non-human primate (NHP) models in preclinical research is also essential since their genetic relatedness to humans makes them a rich source of information, further necessitating the development of specific viral vectors in this field.
Restraining Factor
High expenses, rigorous regulations, ethical concerns, and biosafety risks restrain the viral vectors NHP market development. High production expenses result from elaborate manufacturing and quality control procedures. Rigorous regulations require substantial safety and efficacy testing. Ethical considerations result from animal welfare concerns. Potential biosafety risks, such as unanticipated viral transmission or immune response, discourage scalability and widespread adoption.
Market Segmentation
The global viral vectors for non-human primates market is divided into vector type, type of non-human primates, and application.
Global Viral Vectors for Non-human Primates Market, By Vector Type:
Why did the adeno-associated vectors segment dominate the market in 2024?
The adeno-associated vectors segment dominated the market in 2024 due to researchers’ increased focus on AAV for gene therapy. Their broad tissue targeting ability and capacity for long-lasting gene expression make them ideal for non-human primate studies. Additionally, the rising prevalence of genetic disorders and infectious diseases drove higher demand.
Get more details on this report -
The retroviral vectors segment in the viral vectors for non-human primates market is expected to grow at the fastest CAGR over the forecast period. This is due to their extensive use in gene therapy and disease treatment. Their ability to integrate into host genomes ensures stable gene expression, while improved efficiency, safety, and targeting make them ideal for gene therapy and vaccine research.
Global Viral Vectors for Non-human Primates Market, By Type of Non-human Primates:
What made rhesus macaques the dominant segment in the viral vectors for non-human primates market?
The rhesus macaques held the largest market share due to their close genetic resemblance to humans, sharing approximately 93% of human DNA. This makes them highly valuable for studying human diseases, gene therapy, and vaccine development. Their genetic similarity enables accurate disease modeling and gene therapy testing. Increasing clinical trials involving rhesus macaques are driving higher demand, supporting significant growth in this segment.
The cynomolgus monkey segment in the viral vectors for non-human primates market is expected to grow at the fastest CAGR over the forecast period. This is driven by increased use in gene therapy and vaccine research. Its genetic and physiological similarity to humans makes it essential for biomedical studies, human disease modeling, and developing innovative treatments.
Global Viral Vectors for Non-human Primates Market, By Application:
How does the gene therapy segment dominate the market in 2024?
The gene therapy segment led the viral vectors market for non-human primates, driven by expanding research and development efforts and extensive viral vector use. Due to their close genetic and physiological resemblance to humans, non-human primates are essential in preclinical studies. Viral vectors play a key role by delivering therapeutic genes, making them indispensable tools in gene therapy research involving non-human primates.
The vaccine research segment in the viral vectors for non-human primates market is expected to grow at the fastest CAGR over the forecast period. The vaccine research segment is rapidly growing, as viral vectors allow vaccine testing in animal models before human trials. Non-human primates’ genetic similarity to humans makes them ideal for preclinical vaccine studies, driving increased viral vector use and segment expansion.
Regional Segment Analysis of the Global Viral Vectors for Non-human Primates Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
North America Viral Vectors for Non-human Primates Market Trends

Get more details on this report -
What factors contribute to North America’s dominance in the viral vectors for non-human primates market?
North America dominated the global market for viral vectors for non-human primates in 2024, with the largest revenue share attributed to its excellent research infrastructure. The increased gene therapy pipeline has driven several clinical trials to confirm treatment efficacy. The region's sophisticated facilities and several key industry players facilitate market growth. Major pharmaceutical companies also significantly invest in novel gene therapy research, driving demand for viral vectors. Growing approvals of new gene therapies and increasing interest in gene and cell therapies propel market growth further.
What are the current trends in the U.S. viral vectors market for non-human primates?
The U.S. dominates the North American market for viral vectors of non-human primates due to increased preclinical and gene therapy research. Increased diseases and genetic similarities of rhesus macaques to humans generate demand. Synergies between researchers and companies increase viral vector development opportunities.
Asia Pacific Viral Vectors for Non-human Primates Market Trends
What factors are driving the rapid growth of the viral vectors market in the Asia Pacific for non-human primates?
The rapid growth of the Asian Pacific viral vectors market for non-human primates is due to various major drivers, such as high investment in biotechnology and drug research by economies such as China and India are boosting the market growth. The region's increasing focus on gene therapy and vaccine production, supported by a high incidence of genetic and infectious diseases, further drives demand. Moreover, the presence of a vast population of non-human primates for preclinical research and positive government policies for encouraging biomedical research are major contributors. Local-international collaborative arrangements also promote market growth and innovation.
Why is India becoming important in the viral vectors market for non-human primate research?
India is becoming increasingly significant in the viral vectors market due to rising biotechnology investments, expanding gene therapy research, and the availability of non-human primates for preclinical testing. Favorable government policies and expanding collaborations drive viral vector advancement and market growth in the country.
Why are these trends developing in China’s viral vectors market for non-human primates?
China is leading the regional market owing to robust government support for biopharmaceuticals, favorable regulatory policies for innovative research, and the existence of veteran CDMOs such as Wuxi App Tech, ASKBio, and GenScript, which have GMP-licensed viral vector production capacity.
Why is Japan significant in the viral vectors market for non-human primate research?
Japan plays an important role in the market for viral vectors due to its leading biomedical research, the strong support of the government, and the well-developed pharmaceutical sector. The interest in gene therapy and non-human primate models in the country fuels innovation and demand for virus vectors in R&D.
Europe Viral Vectors for Non-human Primates Market Trends
What factors are driving growth in Europe’s viral vectors market for non-human primates?
Europe is at the forefront of global market action, spurred by the growing use of non-human primates in preclinical disease models for cancer and neurological disorders. Approvals of many gene therapies drive demand for viral vectors. Biopharm companies are moving towards in-house production of viral vectors, spending more to minimize dependency on CDMOs, further propelling regional market growth.
What are the key trends in Germany’s viral vectors market for non-human primates?
Germany is emerging as a leading market force due to robust pharmaceutical firms, multiple gene therapy trials, and sophisticated viral vector production. Intense demand for gene therapies has led to government and popular support, which has translated into tremendous growth in the gene therapy and viral vector market.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global viral vectors for non-human primates market, along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
Worldwide Top Key Players In The Viral Vectors for Non-human Primates Market Include
- Lonza Group AG
- REGENXBIO Inc.
- Oxford Biomedica
- Merck KGaA
- 4D Molecular Therapeutics
- Biovian Oy
- Thermo Fisher Scientific Inc.
- CRISPR Therapeutics
- Barinthus Biotherapeutics
- Genezen
- Andelyn Biosciences
- Fujifilm Diosynth Biotechnologies
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent development
- In May 2025, Andelyn Biosciences manufactured a viral vector using its AAV Curator Platform for Nationwide Children’s Hospital. The AAV supports a novel cell therapy in a Phase 1 trial assessing safety in advanced, high-risk acute myeloid leukemia patients.
- In July 2024, Merck signed a non-binding MoU with Japan-based GTRI, a gene therapy biotech firm, to use Merck’s Sf-RVN Insect Cell Line platform for GMP production of AAV-based gene therapy targeting Parkinson’s disease.
- In February 2024, Andelyn Biosciences was chosen to manufacture adeno-associated virus (AAV) therapies using its suspension platform for multiple programs under the FNIH Accelerating Medicines Partnership Bespoke Gene Therapy Consortium, advancing gene therapy development and patient-focused solutions.
- In September 2023, FUJIFILM Biotechnologies completed a new cGMP facility in Darlington, UK, for manufacturing viral gene therapies, oncolytic viruses, and vaccines, supporting early-stage clinical trials and reflecting the company’s commitment to growing viral vector production demand.
- In October 2022, Lonza announced the expansion of its cell and gene therapy development labs in Houston and Geleen, enhancing global process development services by increasing capabilities and capacity at two of the industry’s largest facilities.
- May 12, 2021, 4D Molecular Therapeutics announced new preclinical data from non-human primate studies of 4D 150, an intravitreal gene therapy targeting four VEGF family members for treating wet AMD and diabetic macular edema using a dual transgene approach.
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the viral vectors for non-human primates market based on the following segments:
Global Viral Vectors for Non-human Primates Market, By Vector Type
- Adenoviral Vectors
- Adeno-associated Vectors
- Retroviral Vectors
- Lentiviral Vectors
- Others
Global Viral Vectors for Non-human Primates Market, By Type of Non–human Primates
- Marmosets
- Rhesus Macaques
- Cynomolgus Monkey
- Others
Global Viral Vectors for Non-human Primates Market, By Application
- Gene Therapy
- Vaccine Research
Global Viral Vectors for Non-human Primates Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the viral vectors for non-human primates market over the forecast period?The global viral vectors for non-human primates market is projected to expand at a CAGR of 10.36% during the forecast period.
-
2. What is the viral vectors for non-human primates market?The viral vectors for non-human primates market involves the development and use of modified viruses to deliver genetic material into non-human primate (NHP) cells for preclinical research and gene therapy testing, driven by NHP relevance in understanding human diseases and advancing treatments.
-
3. What is the market size of the viral vectors for non-human primates market?The global viral vectors for non-human primates market size is expected to grow from USD 143.6 million in 2024 to USD 424.51 million by 2035, at a CAGR 10.36% of during the forecast period 2025-2035.
-
4. What are the market trends in the viral vectors for non-human primates market?Market trends include increased use of viral vectors in NHP preclinical studies for genetic and oncological diseases, advancements in AAV and lentiviral vector technology, and rising demand for gene therapy and vaccine research.
-
5. Which region holds the largest share of the viral vectors for non-human primates market?North America is anticipated to hold the largest share of the viral vectors for non-human primates market over the predicted timeframe.
-
6. What factors are driving the growth of the viral vectors for non-human primates market?Growth is driven by increasing gene therapy demand, advanced preclinical research for complex diseases such as neurodegeneration, and rising regulatory support for cell and gene therapies. Technological innovations, such as improved vector targeting and delivery methods, enhance efficacy.
-
7. What are the main challenges restricting wider adoption of the viral vectors for non-human primates market?Challenges include high production costs, difficulties scaling up manufacturing, potential pre-existing immunity in primates, vector-related toxicity, and the complex translation of research from lower species to primates.
-
8. Who are the top 10 companies operating in the global viral vectors for non-human primates market?The major players operating in the viral vectors for non-human primates market are Lonza Group AG, REGENXBIO Inc., Oxford Biomedica, Merck KGaA, 4D Molecular Therapeutics, Biovian Oy, Thermo Fisher Scientific Inc., CRISPR Therapeutics, Barinthus Biotherapeutics, Genezen, Andelyn Biosciences, Fujifilm Diosynth Biotechnologies, and Others.
Need help to buy this report?