Global Viral Vector And Plasmid DNA Testing Services Market Size, Share, and COVID-19 Impact Analysis, By Testing Services (Safety, Genetic Characterization, Purity, Identity, and Potency), By End-User (Research Organizations and Pharmaceutical and Biotechnological Companies), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2021 - 2030.
Industry: HealthcareGlobal Viral Vector And Plasmid DNA Testing Services Market Insights Forecasts to 2030
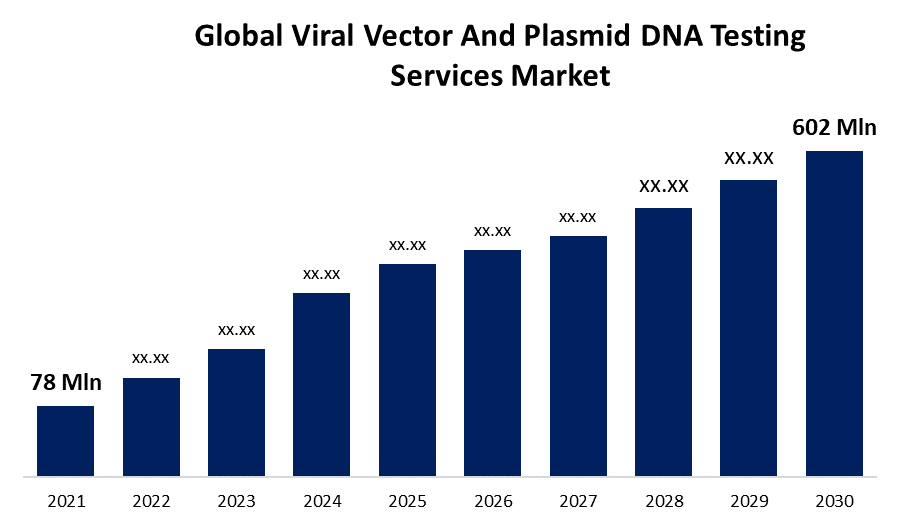
- The Global Viral Vector And Plasmid DNA Testing Services Market Size was valued at USD 78 Million in 2021.
- The Market Size is Growing at a CAGR of 25.5% from 2022 to 2030
- The Worldwide Viral Vector And Plasmid DNA Testing Services Market Size is Expected to reach USD 602 Million by 2030
- The Asia Pacific is Expected to Grow the fastest during the forecast period
Get more details on this report -
The Global Viral Vector And Plasmid DNA Testing Services Market Size is Expected to reach USD 602 Million by 2030, at a CAGR of 25.5% during the forecast period 2022 to 2030.
The Viral vector and plasmid DNA testing services market has grown due to increased research and development. In addition, the wide use of these tools in delivering genetic materials into cells also expands the market growth.
Market Overview
The increased innovation and expenditure in research and development are expected to impact the global market for viral vector and plasmid DNA testing services. Molecular biologists frequently use viral vectors, popular among this group, to introduce genetic material into cells. The exact process can be carried out inside a live thing or in cell culture (in vitro) (in vivo). Viruses have evolved into specialized molecular processes to carry their genomes into the cells they infect. Plasmids, on the other hand, are tiny DNA molecules that may physically split from chromosomal DNA and then replicate on their own.
These creatures, found in bacteria as double-stranded, circular DNA molecules, are commonly used in biotechnology and genetic engineering labs. They are used there to express particular gene types or to amplify and clone certain types of genes. Due to the rising incidence of target ailment and disease and the effectiveness of viral vectors in delivering gene therapy, the market for viral vectors and plasmid DNA testing is expanding. This increase is made possible by funding for the development of gene therapy as well as ongoing research into cell and gene therapies based on viral vectors.
The demand for scalable production of gene therapy vectors is also anticipated to rise due to the number of gene therapy-based discovery initiatives launched by biotechnology and pharmaceutical businesses. The worldwide industry is being driven by an increase in the number of patients choosing gene therapy. The demand for plasmid DNA is soaring as gene therapy research and development picks up steam. Thus, pDNA (Plasmid DNA) production is necessary for Adeno-associated virus (AAV), lentivirus, and other viral vector systems. Additionally, several hereditary disorders and infectious diseases are spreading across the globe. For instance, according to UNAIDS 1.7 million persons acquired HIV in 2019, and 38.0 million people worldwide were living with the disease.
Global Viral vector And Plasmid DNA Testing Services Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2021 |
| Market Size in 2021: | USD 78 Million |
| Forecast Period: | 2022-2030 |
| Forecast Period CAGR 2022-2030 : | 25.5 % |
| 2030 Value Projection: | USD 602 Million |
| Historical Data for: | 2019-2020 |
| No. of Pages: | 260 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Testing Services, By End-User, By Region |
| Companies covered:: | Charles River Laboratories, Inc., WuXi AppTec Co., Ltd., Cobra Biologics and Pharmaceutical Services, Merck KgaA, Lonza, Eurofins Scientific, FinVector Vision Therapies, Advanced Bioscience Laboratories, Inc., Takara Bio Inc., ViruSure GmbH, and Genezen Laboratories. |
| Growth Drivers: | Rise in R&D is expected to drives the markets growth over the forecast period. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Report Coverage
This research report categorizes the market for global viral vector and plasmid DNA testing services based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global Viral vector and plasmid DNA testing services market.
Recent market developments and competitive strategies such as expansion, product launch and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each global Viral vector and plasmid DNA testing services market sub-segments.
Segmentation Analysis
- In 2021, the safety segment dominated the market with the largest market share of 35% and market revenue of 27.3 million.
Based on the testing services, the global Viral vector and plasmid DNA testing services market is categorized into Safety, Genetic Characterization, Purity, Identity, and Potency. In 2021, the safety segment dominated the market with the largest market share of 35% and market revenue of 27.3 million. Because manufacturers are launching more goods and services, the market is likely to develop as a result. To increase the availability of its clinical testing services in the Asia Pacific market, Merck KGaA, for instance, opened the GMP BioReliance biosafety testing laboratory in Singapore in September 2018. Additionally, LabCorp's Covance drug development division introduced a portfolio of cell and gene therapy development products in January 2020. The remedy is created to hasten the advancement of gene and cell treatment. Under this, Labcorp provides clinical and preclinical solutions, including safety evaluation and bioanalysis testing and services.
- In 2021, the research organizations segment accounted for the largest share of the market, with 70% and a market revenue of 54.6 million.
Based on end-user, the Viral vector and plasmid DNA testing services market is categorized into Research Organizations and Pharmaceutical and Biotechnological Companies. In 2021, the research organizations segment accounted for the largest share of the market, with 70% and a market revenue of 54.6 million. Due to the high demand for viral vectors for conducting research, it is anticipated that the growing involvement of scientific communities in gene and cell therapy research would boost the demand for viral vectors. In addition, the market for viral vectors and plasmid DNA testing is seeing the emergence of research institutions as major end-users, which is helping to drive the development of sophisticated drugs and an increasing number of gene therapy-based R&D initiatives. One of these companies, Abeona Therapeutics, for instance, is exploring AAV9-based gene therapies for CLN1 and CLN3 illnesses. It will therefore encourage market expansion.
Regional Segment Analysis of the Viral Vector And Plasmid DNA Testing Services Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)

Get more details on this report -
North America emerged as the largest market for the global Viral vector and plasmid DNA testing services market, with a market share of around 34.1% and 78 million of the market revenue in 2021.
- In 2021, North America emerged as the largest market for the global Viral vector and plasmid DNA testing services market, with a market share of around 34.1% and 78 million of the market revenue. North America is expected to be the largest market. The existence of a sizable number of centers and institutes involved in the R&D of advanced medicines is one of the key factors that has contributed to the huge share of this regional market. The federal agencies' investments in the region's cell therapy research base are expected to boost the market's expansion in North America.
- The Asia-Pacific market is expected to grow at the fastest CAGR between 2021 and 2030, owing to the growing patient population, the expansion of R&D, and other factors. Additionally, because the legal environment is less severe in this location, patients from western nations are flying there for stem cell therapy. Global businesses have also changed their business strategies in this region as a result of the enormous regional population and untapped potential. Additionally, this area provides reasonably priced operating & manufacturing facilities for performing research. These elements are anticipated to significantly contribute to the development of the viral vector and plasmid DNA testing services sector in this area and accelerate the market's expansion.
Competitive Landscape
The report offers the appropriate analysis of the key organizations/companies involved within the global Viral vector and plasmid DNA testing services market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the companies' current news and developments, including product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Market Players:
- Charles River Laboratories, Inc.
- WuXi AppTec Co., Ltd.
- Cobra Biologics and Pharmaceutical Services
- Merck KgaA
- Lonza
- Eurofins Scientific
- FinVector Vision Therapies
- Advanced Bioscience Laboratories, Inc.
- Takara Bio Inc.
- ViruSure GmbH
- Genezen Laboratories
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Third-party knowledge providers
- Value-Added Resellers (VARs)
Some of the Key Developments:
- In July 2021, Thermo Fisher Scientific Inc. announced the opening of a new cGMP plasmid DNA manufacturing facility in Carlsbad, California, to meet the rising demand for plasmid DNA-based medicines and essential mRNA-based vaccines.
- In February 2021, Wacker stated that it has acquired Genopis Inc., a significant plasmid DNA producer based in the US.
Market Segment
This study forecasts global, regional, and country revenue from 2019 to 2030. Spherical Insights has segmented the global Viral vector and plasmid DNA testing services market based on the below-mentioned segments:
Global Viral vector and plasmid DNA testing services market, By Testing Services
- Safety
- Genetic Characterization
- Purity
- Identity
- Potency
Global Viral vector and plasmid DNA testing services market, By End-User
- Research Organizations
- Pharmaceutical and Biotechnological Companies
Global Viral vector and plasmid DNA testing services market, Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of Middle East & Africa
Frequently Asked Questions (FAQ)
-
What is the market size of the Viral Vector and Plasmid DNA Testing Services market?As per Spherical Insights, the size of the Viral Vector and Plasmid DNA Testing Services market was valued at USD 78 million in 2022 to USD 602 million by 2030.
-
What is the market growth rate of the Viral Vector and Plasmid DNA Testing Services market?The Viral Vector and Plasmid DNA Testing Services market is growing at a CAGR of 25.5% from 2022 to 2030.
-
Which country dominates the Viral Vector and Plasmid DNA Testing Services market?North America emerged as the largest market for Viral Vector and Plasmid DNA Testing Services.
-
Who are the key players in the Viral Vector and Plasmid DNA Testing Services market?Key players in the Viral Vector and Plasmid DNA Testing Services market are Charles River Laboratories, Inc., WuXi AppTec Co., Ltd., Cobra Biologics and Pharmaceutical Services, Merck KgaA, Lonza, Eurofins Scientific, FinVector Vision Therapies, Advanced Bioscience Laboratories, Inc., Takara Bio Inc., ViruSure GmbH, and Genezen Laboratories.
-
Which factor drives the growth of the Viral Vector and Plasmid DNA Testing Services market?Rise in R&D is expected to drives the market's growth over the forecast period.
Need help to buy this report?