The United States Biosimilar Monoclonal Antibodies Market Size, Share, and COVID-19 Impact Analysis, By Product (Infliximab, Rituximab, Abciximab, Trastuzumab, Adalimumab, Bevacizumab, and Others), By Application (Oncology, Chronic & Autoimmune Diseases, and Others), and United States Biosimilar Monoclonal Antibodies Market Insights, Industry Trend, Forecasts to 2035.
Industry: HealthcareThe United States Biosimilar Monoclonal Antibodies Market Insights Forecasts To 2035
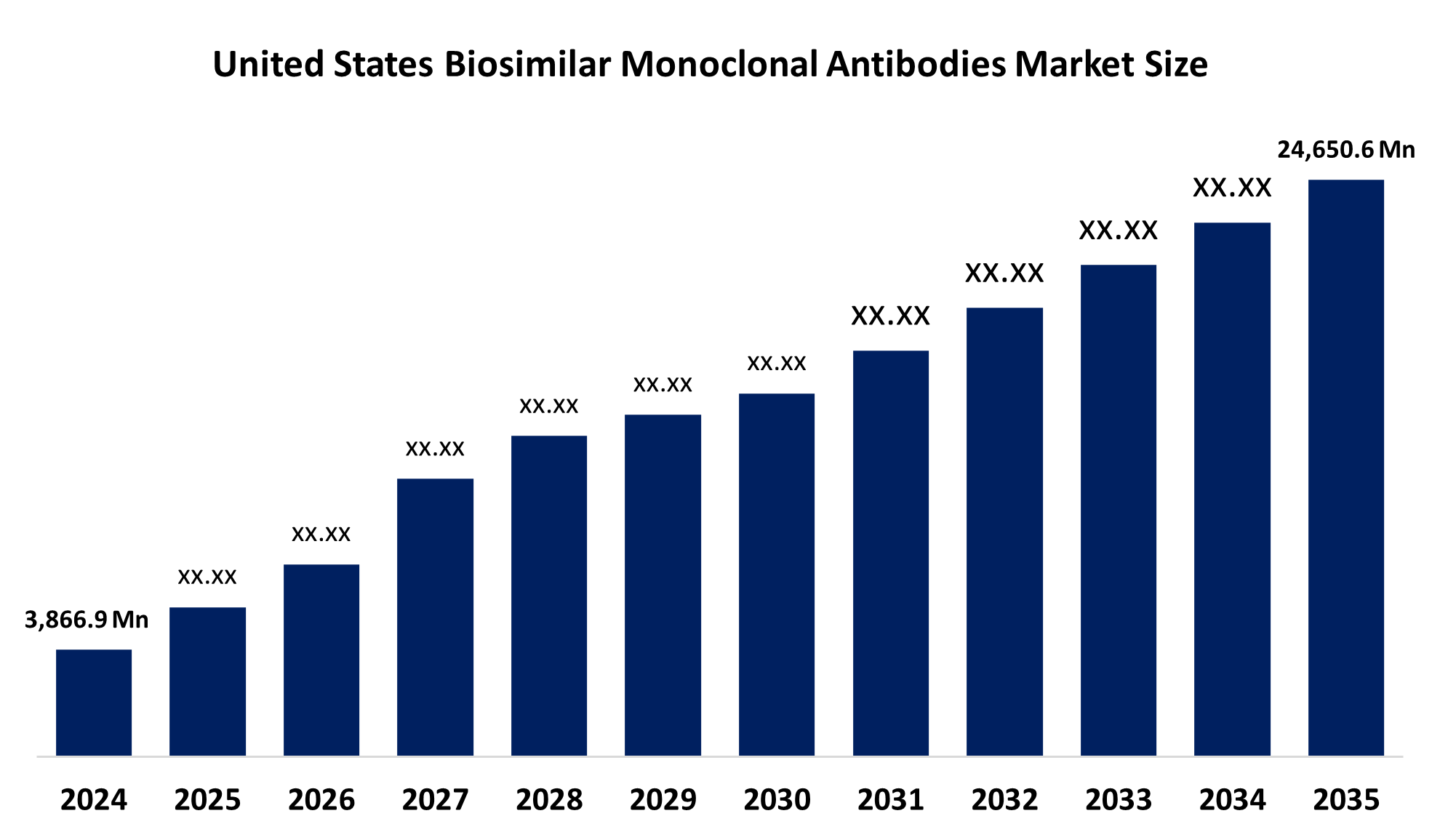
- The United States Biosimilar Monoclonal Antibodies Market Size Was Estimated at USD 3,866.9 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 18.34% from 2025 to 2035
- The United States Biosimilar Monoclonal Antibodies Market Size is Expected to Reach USD 24,650.6 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the US biosimilar monoclonal antibodies Market is anticipated to reach USD 24,650.6 million by 2035, growing at a CAGR of 18.34% from 2025 to 2035. The growing prevalence of autoimmune and chronic diseases like cancer and rheumatoid arthritis, the increasing patent expirations of monoclonal antibodies, the development of mAbs, and the growing need for affordable products
Market Overview
Biosimilar monoclonal antibody biologic medicinal products are similar to a reference monoclonal antibody that has already received approval in the context of composition, function, safety, purity, and efficacy. Biosimilar is obtained from living organisms and, due to their complex molecular structures, cannot be completely repeated like generic pharmaceuticals, which are chemically similar copies of small-molecule drugs. According to regulatory bodies such as the Food and Drug Administration (FDA), there should be no clinically significant changes in medical results from the original biologic. There are many advantages for healthcare in the use of biosimilar monoclonal antibodies, such as an increase in competition among producers, better access to treatment, and financial savings for individuals and healthcare systems. For instance, biosimilar monoclonal antibodies have entered markets worldwide for blockbuster biologics such as infliximab, rituximab, abciximab, trastuzumab, adalimumab, and bevacizumab, which are driving powerful advances in oncology and immunology therapies. BIOSIMIR MABS gives pharmaceutical companies the opportunity to diversify their product lines, increase market competition, and increase the patient's reach in response to the increasing need of biological medicines in oncology, autoimmune, and chronic diseases.
Report Coverage
This research report categorizes the market for the US biosimilar monoclonal antibodies market based on various segments and regions and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the USA biosimilar monoclonal antibodies market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the U.S. biosimilar monoclonal antibodies market.
Driving Factors
The United States biosimilar monoclonal antibodies are driven by autoimmune disorders and the increased prevalence of cancer, blockbuster drug patent termination, and the need for considerably priced biological drugs increases. Strong possibilities for pharmaceutical companies to broaden their portfolio and market access are being created by the rapid adoption of biosimilars, which are being expedited by the assistant regulatory structure, healthcare system cost-control objectives, and increasing physician and patient acceptance of these products.
Restraining Factors
The United States biosimilar monoclonal antibodies are restrained by strict regulatory restrictions and high manufacturing complexity. Wide adoption and delay in some areas may be interrupted by the patient's anxiety, cost pressure, and competition from branded biologics due to limited physician awareness, safety, and efficacy.
Market Segmentation
The United States biosimilar monoclonal antibodies market share is classified into product and application.
- The infliximab segment accounted for the largest market share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period.
The United States biosimilar monoclonal antibodies market is segmented by product into infliximab, rituximab, abciximab, trastuzumab, adalimumab, bevacizumab, and others. Among these, the infliximab segment accounted for the largest market share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. The increasing number of people with autoimmune conditions, including Crohn's disease and rheumatism, has increased the demand for biosimilar infliximab as an easily available treatment option, promoting the growth of the market.
- The oncology segment held the largest revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The United States biosimilar monoclonal antibodies market is segmented by application into oncology, chronic & autoimmune diseases, and others. Among these, the oncology segment held the largest revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period. Increase in the prevalence of various cancers and escalating need for economical yet potent treatments for cancer. Biosimilar monoclonal antibodies present comparable efficacy and safety profiles to their originator counterparts but at a more affordable cost. This is facilitated by factors such as patent expirations, regulatory pathways that support biosimilars, and increasing confidence in their clinical performance.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the United States biosimilar monoclonal antibodies market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Pfizer Inc.
- Amgen Inc.
- Merck & Co., Inc.
- Abbott Laboratories
- Eli Lilly and Company
- Johnson & Johnson Services, Inc.
- Bristol Myers Squibb
- Thermo Fisher Scientific, Inc
- Others
Key Target Audience
- Market Players
- Investors
- End users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value Added Resellers (VARs)
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the United States biosimilar monoclonal antibodies Market based on the below-mentioned segments:
US Biosimilar Monoclonal Antibodies Market, By Product
- Infliximab
- Rituximab
- Abciximab,
- Trastuzumab
- Adalimumab
- Bevacizumab
- Others
US Biosimilar Monoclonal Antibodies Market, By Application
- Oncology
- Chronic & Autoimmune Diseases
- Others
Frequently Asked Questions (FAQ)
-
1. What was the market size of the United States biosimilar monoclonal antibodies market in 2024?The United States biosimilar monoclonal antibodies market was valued at USD3,866.9 million in 2024.
-
2. What is the expected market size by 2035?The market is expected to grow at a CAGR of 18.34% during the forecast period.
-
3. What are biosimilar monoclonal antibodies?They are biologic medicines highly similar to approved reference monoclonal antibodies in safety, purity, and efficacy, but offered at lower costs.
-
4. Which product held the largest share in 2024?The infliximab segment accounted for the largest market share in 2024.
-
5. Which application dominates the U.S. market?The oncology segment held the largest revenue share in 2024 due to the rising prevalence of cancer.
-
6. What are the key driving factors for market growth?Patent expirations of biologics, rising prevalence of cancer and autoimmune diseases, demand for affordable biologics, and supportive FDA regulatory frameworks.
-
7. What are the main restraining factors?High manufacturing complexity, strict regulatory requirements, safety concerns, and competition from branded biologics.
-
8. Who are the major players in the U.S. market?Key companies include Pfizer, Amgen, Merck & Co., Abbott Laboratories, Eli Lilly, Johnson & Johnson, Bristol Myers Squibb, Viatris, Biogen, and Thermo Fisher Scientific and Others.
-
9. What is the forecast period covered in the report?The study covers 2020–2035, with 2024 as the base year and 2025–2035 as the forecast years.
Need help to buy this report?