United Kingdom Real World Evidence Solutions Market Size, Share By Application (Medical Device Development & Approvals, Reimbursement/Coverage & Regulatory Decision Making, Post Market, Safety & Adverse Events Monitoring, and Drug Development & Approvals), By End Use (Healthcare Companies, Commercial, HEOR, Clinical research, Healthcare Providers, and Healthcare Payers), United Kingdom Real World Evidence Solutions Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareUnited Kingdom Real World Evidence Solutions Market Insights Forecasts to 2035
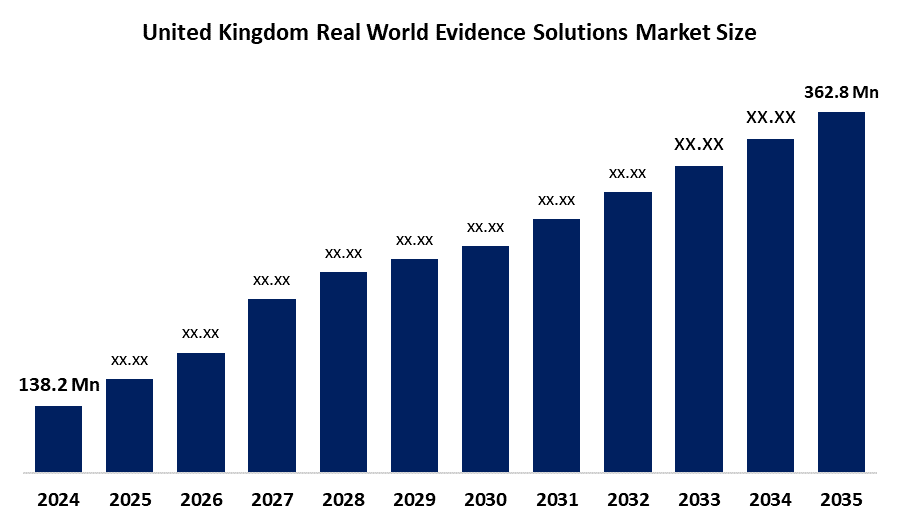
- United Kingdom Real World Evidence Solutions Market Size 2024: USD 138.2 Mn
- United Kingdom Real World Evidence Solutions Market Size 2035: USD 362.8 Mn
- United Kingdom Real World Evidence Solutions Market CAGR 2024: 9.17%
- United Kingdom Real World Evidence Solutions Market Segments: Application and End Use
Get more details on this report -
The real-world evidence (RWE) solutions market in the UK is a growing component of the broader healthcare and life sciences industry. The RWE solutions market consists of the collection of real-world data, real-world evidence analytics platforms, real-world evidence evidence generation services and solutions for post-market monitoring of medical products and services, all of these are enabled by electronic health records, clinical registries, claims data and patient-reported outcomes. RWE solutions can now be used for researching many aspects of pharmaceutical and medical device development, and the digital health ecosystem in supporting regulatory approvals, the assessment of health technology and the development of value-based models for the delivery of healthcare. The RWE Solutions market continues to grow in the UK and is driven by increases in the number of older people, the increase in the prevalence of chronic diseases, and an increased demand for low-cost outcomes of services being provided by NHS England. Strong data assets created by the NHS, pressure to optimise clinical pathways, and the shortening of timeframes to market are also contributing to increased adoption of RWE Solutions.
The UK government, through the MHRA, launched the Real-World Evidence Scientific Dialogue Programme to support innovators via confidential regulatory engagement. The pilot provides guidance on RWE generation and includes MHRA–NICE “safe harbour” workshops, aligning regulatory and health technology assessment expectations while strengthening the UK real world evidence ecosystem.
The growing market for UK-based Real World Evidence (RWE) project solutions offers significant opportunity for growth with increased rates of chronic diseases, an ageing population and increased pressure on the National Health Service (NHS) to provide evidence of both clinical and economic value. The ongoing demand for real time outcomes data from pharmaceutical companies and medical device manufacturers to support their applications for regulatory approvals, pricing and reimbursement decisions is driving the growing use of RWE solutions. There has also been a significant increase in digital health record technology, methods for linking different types of health-related records, new technology and advancements in data analytics as well as artificial intelligence that all provide additional opportunities for creating scalable RWE Platforms to create RWE solutions. Some of the key areas with the greatest potential for growth include post-market surveillance, Health Technology Assessment support, Comparative Effectiveness Research and Value-dependant Analytics related to Value-Based Care, and Government Programs designed to support development of integrated data sets, collaboration between the NHS and Industry, and Life Sciences Innovation, all of which continue to be challenged by data Governance, Interoperability, Access to a highly skilled workforce.
United Kingdom Real World Evidence Solutions Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 138.2 Million |
| Forecast Period: | 2024-2035 |
| Forecast Period CAGR 2024-2035 : | 9.17% |
| 2035 Value Projection: | USD 362.8 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 140 |
| Tables, Charts & Figures: | 102 |
| Segments covered: | By Application,By End Use |
| Companies covered:: | IQVIA Holdings Inc., IBM (Merative), Thermo Fisher Scientific (PPD), PAREXEL International, PerkinElmer, ICON plc, Oracle Corporation, Syneos Health, and Other Key Players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysi |
Get more details on this report -
Market Dynamics of the United Kingdom Real World Evidence Solutions Market
The market for real-world evidence (RWE) solutions in the United Kingdom is developing rapidly with the support of the UK Government, through initiatives aimed at developing Life Sciences and Data Driven Health Policy, as well as through the growth of collaboration between NHS organisations and industry to enhance the health of both the elderly and those suffering from many chronic diseases. Furthermore, RWE is being adopted more widely across the Pharmaceutical, Medical Device, and Digital Health Industries as a result of this shift towards Value Based Health Care, as well as greater efficiency in regulatory decision making and how resources are being utilised. Investment in NHS Data Platforms, Data Linkage, Population Health Analytics, and AI Powered RWE Generation, will further enhance the ability of all regulatory authorities, payers, and manufacturers to assess RWE for its real world efficacy and safety. This enhanced capacity has been achieved through the creation of Government-backed initiatives by the MHRA and NICE, as well as through Public-Private Partnerships, Advanced Analytics, and Interoperable Health Datasets.
The United Kingdom Real World Evidence Solutions Market Size faces restraints includes data access and interoperability challenges, complex data governance and privacy regulations, and variability in data quality across NHS sources. Skills shortages in data science and health analytics, high infrastructure costs, and lengthy approval processes can slow adoption, while budget pressures, procurement complexity, and uneven organizational readiness across regions affect market scalability, despite strong long-term demand for RWE in regulatory, payer, and clinical decision-making.
The Future of the United Kingdom Real World Evidence Solutions Market Size together with the advancements in Innovation Technology for Health and Life Sciences, Policies that Support the Health and Life Sciences Sector, as well as an increase in Demand for RWE in Health Care through Data-Driven Decision Making. New Sources of Growth Continue to Emerge within Pharmaceuticals, Medical Devices and Population Health through Use of New Technology Such as Advanced Analytical Techniques, Artificial Intelligence, Real-Time Data Linking, and Integrated Health Records. In Support of RWE Growth are NHS Data Assets, Stronger Acceptance of RWE by the MHRA and the NICE as Evidence for Value, and Increased Private/Public Collaboration to Support RWE Generation.
Market Segmentation
The United Kingdom Real World Evidence Solutions Market share is classified into application and end use.
By Application
The United Kingdom Real World Evidence Solutions Market Size is divided by application into medical device development & approvals, reimbursement/coverage & regulatory decision making, post market, safety & adverse events monitoring, drug development & approvals. Among these, the drug development & approvals segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. It is driven by its crucial role in speeding up drug creation, reducing costs, and meeting growing demands for faster regulatory approvals.
By End Use
The United Kingdom Real World Evidence Solutions Market Size is divided by end use into healthcare companies, commercial, heor, clinical research, healthcare providers, and healthcare payers. Among these, the healthcare companies segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. It is driven by their need to support drug development, gain regulatory approvals (like from MHRA), understand post-market performance, and secure market access.
Competitive Analysis
The report offers the appropriate analysis of the key organisations/companies involved within the United Kingdom Real World Evidence Solutions Market Size, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
Top Key Companies in United Kingdom Real World Evidence Solutions Market
- IQVIA Holdings Inc.
- IBM (Merative)
- Thermo Fisher Scientific (PPD)
- PAREXEL International
- PerkinElmer
- ICON plc
- Oracle Corporation
- Syneos Health
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the United Kingdom, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the United Kingdom Real World Evidence Solutions Market Size based on the below-mentioned segments:
United Kingdom Real World Evidence Solutions Market, By Application
- Medical Device Development & Approvals
- Reimbursement/Coverage & Regulatory Decision Making
- Post Market, Safety & Adverse Events Monitoring
- Drug Development & Approvals
United Kingdom Real World Evidence Solutions Market, By End Use
- Healthcare Companies
- Commercial
- HEOR
- Clinical research
- Healthcare Providers
- Healthcare Payers
Frequently Asked Questions (FAQ)
-
What is the United Kingdom real world evidence solutions market size?United Kingdom real world evidence solutions market is expected to grow from USD 138.2 million in 2024 to USD 362.8 million by 2035, growing at a CAGR of 9.17% during the forecast period 2025-2035.
-
What are the key growth drivers of the market?Market growth is driven by initiatives aimed at developing Life Sciences and Data Driven Health Policy, as well as through the growth of collaboration between NHS organisations and industry to enhance the health of both the elderly and those suffering from many chronic diseases.
-
What factors restrain the United Kingdom real world evidence solutions market?Constraints include the data access and interoperability challenges, complex data governance and privacy regulations, and variability in data quality across NHS sources.
-
How is the market segmented by application?The market is segmented into medical device development & approvals, reimbursement/coverage & regulatory decision making, post market, safety & adverse events monitoring, drug development & approvals.
-
Who are the key players in the United Kingdom real world evidence solutions market?Key companies include IQVIA Holdings Inc., IBM (Merative), Thermo Fisher Scientific (PPD), PAREXEL International, PerkinElmer, ICON plc, Oracle Corporation, and Syneos Health, and Others.
-
Who are the target audiences for this market report?The report targets market players, investors, end-users, government authorities, consulting and research firms, venture capitalists, and value-added resellers (VARs).
Need help to buy this report?