United Kingdom Extractable and Leachable Testing Services Market Size, Share, By Product (Single-use System, Container Closure Systems, and Drug Delivery System), By Application (Ophthalmic, Parenteral Drug Products, Orally Inhaled and Nasal Drug Products), and United Kingdom Extractable and Leachable Testing Services Market Insights, Industry Trend, Forecasts to 2035.
Industry: HealthcareUnited Kingdom Extractable and Leachable Testing Services Market Insights Forecasts to 2035
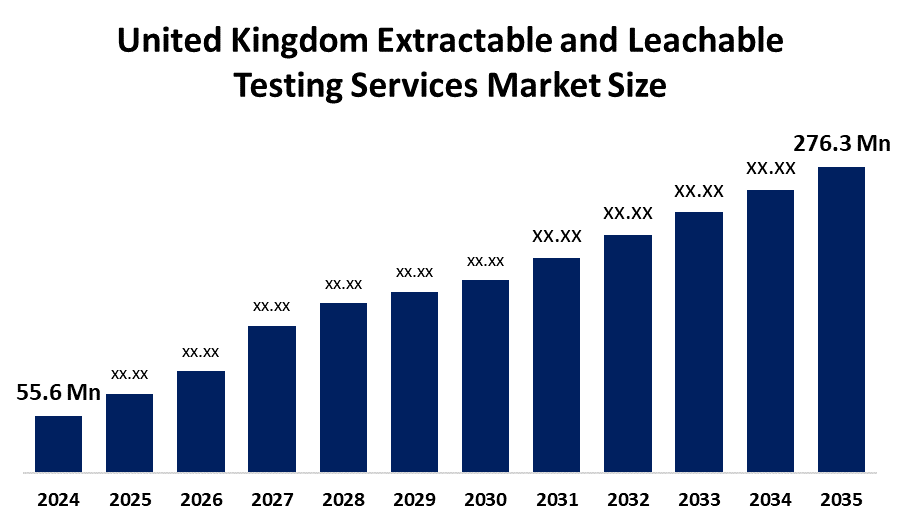
- United Kingdom Extractable and Leachable Testing Services Market Size 2024: USD 54.6 Mn
- United Kingdom Extractable and Leachable Testing Services Market Size 2035: USD 276.3 Mn
- United Kingdom Extractable and Leachable Testing Services Market CAGR: 15.88%
- United Kingdom Extractable and Leachable Testing Services Market Segments: Product and Application
Get more details on this report -
The United Kingdom extractable and leachable (E&L) testing services market is expanding in response to strong demand from pharmaceutical, biotechnology, and medical device sectors. To guarantee product safety, stability, and regulatory compliance, market data shows rising investment in biologics, parenteral medications, single-use systems, and innovative delivery platforms, all of which call for thorough E&L evaluations. Outsourced analytical testing services are continuously expanding due to increased drug development activities and strict quality criteria.
The UK government supports this ecosystem through broader life sciences and regulatory initiatives that strengthen the country’s attractiveness for clinical and manufacturing activities. The need for high-quality testing services, including E&L studies, is maintained by policies targeted at increasing pharmaceutical R&D, simplified regulatory frameworks following Brexit that are in line with international standards (MHRA, EMA), and incentives for innovation. Analytical science capabilities are also enhanced via funding programs and partnerships between industry and public research organizations.
Technological advancement is a key enabler of market growth. The sensitivity, throughput, and dependability of E&L testing are increased by the use of high-resolution analytical platforms (such as LC-MS, GC-MS, and ICP-MS), improved data interpretation software, and automated sample preparation. By combining machine learning and informatics, risk assessment and regulatory reporting can be completed more quickly and accurately, putting UK labs at the forefront of compliance extractable and leachable analysis.
United Kingdom Extractable and Leachable Testing Services Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 54.6 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR 15.88% |
| 2035 Value Projection: | USD 276.3 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 132 |
| Segments covered: | By Product,By Application |
| Companies covered:: | Eurofins Scientific, Intertek Group plc, SGS SA, Smithers, WuXi AppTec, Nelson Labs, Merck KGaA, Others |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Market Dynamics of the United Kingdom Extractable and Leachable Testing Services Market:
The pharmaceutical, biotechnology, and medical device industries, particularly the growing development of injectables, biologics, combination medicines, and sophisticated drug delivery systems are driving the market for extractable and leachable (E&L) testing services in the United Kingdom. E&L studies for medical devices, single-use technologies, and container closing systems are required by strict regulatory authorities, including the MHRA, EMA, FDA, and USP. Further driving market expansion are the expanding usage of single-use systems in biomanufacturing, the outsourcing of analytical testing to specialized labs, and the increased emphasis on patient safety and product quality.
The market is subject to some limitations despite powerful drivers. Adoption may be constrained, particularly among small and mid-sized enterprises, by high testing costs, intricate and time-consuming research designs, and the requirement for sophisticated analytical equipment like LC-MS, GC-MS, and ICP-MS. Operational difficulties are further compounded by a lack of highly qualified analytical specialists and changing regulatory requirements.
However, the market presents significant opportunities. The demand for E&L testing services is rising due to increased investment in biologics, biosimilars, inhalation medicines, and parenteral formulations. In addition to increasing cooperation between pharmaceutical companies and contract research organizations, technological developments in high-resolution mass spectrometry, automation, and data analytics are anticipated to open up new growth opportunities and solidify the UK's standing as a major hub for regulatory-compliant E&L testing services.
Market Segmentation
The United Kingdom extractable and leachable testing services market share is classified into product and application.
By Product:
Based on product, the United Kingdom extractable and leachable testing services market is divided into single-use systems, container closure systems, and drug delivery systems. Among these, the container closure systems segment accounted for the largest market share in 2024 and is expected to grow at a significant rate of CAGR during the projected period. Increasing production of biologics, injectables, and high-value pharmaceuticals, along with stringent regulatory requirements from agencies such as the MHRA and EMA, is compelling manufacturers to conduct comprehensive E&L studies, thereby supporting the sustained growth of this segment.
By Application:
The United Kingdom extractable and leachable testing services market is classified by application into ophthalmic, parenteral drug products, orally inhaled and nasal drug products. Among these, the parenteral drug products segment held the largest market share in 2024 and is expected to grow at a remarkable CAGR during the forecast period. The large number of injectable formulations that necessitate stringent E&L testing because of their direct interaction with container closure mechanisms and single-use components is the main factor driving this leadership.
Competitive Analysis:
The report offers the appropriate analysis of the key organisations/companies involved within the United Kingdom extractable and leachable testing services market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
Top Key Companies in the United Kingdom Extractable and Leachable Testing Services Market:
- Eurofins Scientific
- Intertek Group plc
- SGS SA
- Smithers
- WuXi AppTec
- Nelson Labs
- Merck KGaA
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the United Kingdom, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the United Kingdom extractable and leachable testing services market based on the following segments:
United Kingdom Extractable and Leachable Testing Services Market, By Product
- Single-use System
- Container Closure Systems
- Drug Delivery System
United Kingdom Extractable and Leachable Testing Services Market, By Application
- Ophthalmic
- Parenteral Drug Products
- Orally Inhaled and Nasal Drug Products
Frequently Asked Questions (FAQ)
-
1. What is the expected market size of the UK extractable and leachable testing services market by 2035?The market is projected to grow from USD 54.6 million in 2024 to USD 276.3 million by 2035, registering a CAGR of 15.88% during 2025–2035.
-
2. What factors are driving the growth of this market?Key growth drivers include rising demand for biologics and injectables, increased adoption of single-use systems, strict regulatory requirements from MHRA, EMA, FDA, and USP, and growing outsourcing of analytical testing services.
-
3. Who are the key players in the UK extractable and leachable testing services market?Major players include Eurofins Scientific, Intertek Group plc, SGS SA, Smithers, WuXi AppTec, Nelson Labs, and Merck KGaA, among others.
Need help to buy this report?