South Korea Point of Care Sepsis Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Product Type (Instruments and Assay Kits & Reagents), By Technology (Immunoassays, Molecular Diagnostics, Biomarker-Based Tests, and Others), and South Korea Point-of-Care Sepsis Diagnostics Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareSouth Korea Point of Care Sepsis Diagnostics Market Insights Forecasts to 2035
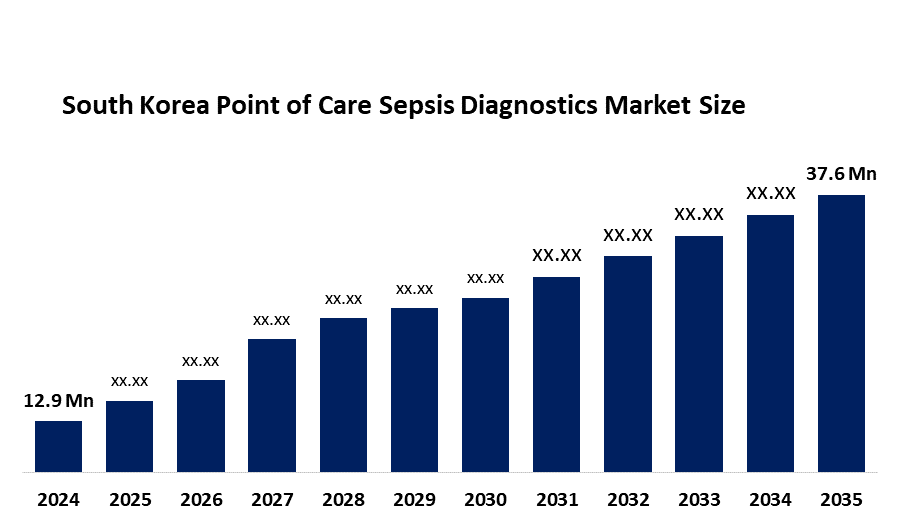
- The South Korea Point of Care Sepsis Diagnostics Market Size Was Estimated at USD 12.9 Million in 2024
- The Market Size is Expected to Grow at a CAGR of Around 10.2% from 2025 to 2035
- The South Korea Point of Care Sepsis Diagnostics Market Size is Expected to Reach USD 37.6 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, The South Korea Point Of Care Sepsis Diagnostics Market Size is anticipated to reach USD 37.6 Million by 2035, Growing at a CAGR of 10.2% from 2025 to 2035. The South Korea point of care sepsis diagnostics market is driven by the rising prevalence of sepsis, increasing demand for rapid diagnostic solutions, growing adoption of point-of-care testing in emergency and critical care settings, and continuous technological advancements in diagnostic platforms.
Market Overview
South Korea point of care sepsis diagnostics market can be defined as the market that involves the development, manufacture, and distribution of rapid diagnostic products for the diagnosis of sepsis at or near the point of care. The point of care sepsis diagnostics market is driven by factors such as the increasing healthcare expenditure, increasing awareness regarding the importance of early sepsis diagnosis, increasing geriatric population, and the development of critical care infrastructure in South Korea. In addition, the point of care sepsis diagnostics market is driven by factors such as increasing hospital readiness, adoption of advanced medical technologies, and efforts to reduce the turnaround time for diagnosis. The point of care sepsis diagnostics market in South Korea is expected to register significant growth due to the increasing awareness regarding the importance of early sepsis diagnosis.
The South Korea point of care sepsis diagnostics market is undergoing three prominent trends that are likely to affect the market in the future. There is a rising trend of early diagnosis of sepsis, as medical professionals are attempting to reduce the mortality rate linked with sepsis. The adoption of biomarker tests and molecular diagnostics is on the rise due to their high sensitivity and ability to deliver results in a matter of minutes. The adoption of digital health technology and AI-based diagnostic tools is improving the efficiency of tests in emergency settings.
The South Korean government is encouraging the point of care diagnostics market through modernization programs in the healthcare sector, infectious disease preparedness strategies, and hospital infrastructure development. The country’s policies that focus on early disease detection and swift response to serious infections are driving the adoption of point of care diagnostic solutions. Microfluidics, biosensors, multiplexing, and automated diagnostic solutions are being increasingly incorporated into sepsis diagnostics. Such advancements improve the accuracy of tests, simplify operations, and facilitate rapid diagnosis, thus positively influencing market growth and competitiveness.
Report Coverage
This research report categorizes the market for the South Korea point of care sepsis diagnostics market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the South Korea point of care sepsis diagnostics market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the South Korea point of care sepsis diagnostics market.
South Korea Point of Care Sepsis Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 12.9 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR of 10.2% |
| 2035 Value Projection: | USD 37.6 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 210 |
| Tables, Charts & Figures: | 95 |
| Segments covered: | By Product Type, By Technology |
| Companies covered:: | Boditech Med Inc., SD Biosensor, Inc., Seegene, Inc., NanoEntek & Sugentech, Inc., Humasis CO., Ltd., Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, Others, and Key Players. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The South Korea point of care sepsis diagnostics market is mainly driven by the increasing incidence of sepsis and blood infections, which demand early and accurate diagnosis. The demand for early-stage diagnosis and consequent clinical intervention is thus fueling the demand for point of care diagnostics. The increasing number of ICUs, the aging population, and the increasing incidence of chronic diseases are also fueling the market. Development of rapid, highly sensitive, user-friendly POC kits, such as those utilizing biomarkers (procalcitonin, cytokines) or molecular diagnostics, allows for quicker results compared to traditional blood cultures. In addition, advancements in technology and increasing awareness among healthcare professionals about the management of sepsis are also fueling the market.
Restraining Factors
The market faces restraints from the high cost of advanced diagnostic devices, which can limit adoption in smaller healthcare facilities. Inconsistent reimbursement policies and regulatory complexities also restrict market growth. Additionally, concerns related to diagnostic accuracy and the need for skilled personnel to operate advanced point-of-care systems may hinder widespread adoption.
Market Segmentation
The South Korea Point of Care Sepsis Diagnostics market share is classified into product type and technology.
- The assay kits & reagents segment accounted for the largest revenue market in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea point of care sepsis diagnostics market is segmented by product type into instruments and assay kits & reagents. Among these, the assay kits & reagents segment accounted for the largest revenue market in 2024 and is expected to grow at a significant CAGR during the forecast period. Owing to their repeated and frequent use. In contrast to the use of diagnostic tools, which are one-time capital expenditures, assay kits and reagents are consumables that are required for every sepsis test and repeated biomarker monitoring, thereby resulting in higher revenue generation. The increasing number of sepsis tests performed in critical care settings further cements the supremacy of this segment.
- The biomarker-based tests segment accounted for the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period.
On the basis of technology, the South Korea point of care sepsis diagnostics market is segmented by immunoassays, molecular diagnostics, biomarker-based tests, and others. Among these, the biomarker-based tests segment accounted for the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period. With the support of high clinical relevance, rapid turnaround time, and broad use in point of care testing, biomarkers such as procalcitonin (PCT), C-reactive protein (CRP), and lactate are widely used for the early diagnosis of sepsis, thus contributing to the high volume of tests being performed compared to molecular diagnostics. Even though immunoassays led the market in the early years because of their established market, the shift towards faster and more sensitive biomarker testing has helped this segment lead in revenue.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the South Korea point of care sepsis diagnostics market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Boditech Med Inc.
- SD Biosensor, Inc.
- Seegene, Inc.
- NanoEntek & Sugentech, Inc.
- Humasis CO., Ltd.
- Abbott Laboratories
- Roche Diagnostics
- Siemens Healthineers
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments:
In Jan 2025, the digital medical products act effective January 2025, aims to streamline approval for AI-based diagnostics, including sepsis predictors.
In April 202, Launched AFIAS MxA/CRP, a POC platform that can differentiate between viral and bacterial infections in 12 minutes.
Market Segment
This study forecasts revenue at the South Korea, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the South Korea Point-of-Care Sepsis Diagnostics market based on the below-mentioned segments:
South Korea Point of Care Sepsis Diagnostics Market, By Product Type
- Instruments
- Assay Kits & Reagents
South Korea Point of Care Sepsis Diagnostics Market, By Technology
- Immunoassays
- Molecular Diagnostics
- Biomarker-Based Tests
- Others
Frequently Asked Questions (FAQ)
-
1. What is the South Korea Point of Care Sepsis Diagnostics market?The market refers to rapid diagnostic technologies used for early detection of sepsis at or near the patient site in South Korea.
-
2. What is the South Korea Point of Care Sepsis Diagnostics market Size?South Korea Point of Care Sepsis Diagnostics market size is expected to grow from USD 12.9 million in 2024 to USD 37.6 million by 2035, growing at a CAGR of 10.2% during the forecast period 2025-2035.
-
3. What are the key drivers of the South Korea Point of Care Sepsis Diagnostics market?Rising sepsis prevalence, demand for rapid diagnosis, technological advancements, and expanding critical care infrastructure.
-
4. Which product types dominate the South Korea Point of Care Sepsis Diagnostics market?Assay kits & reagents and biomarker-based diagnostics dominate the market.
-
5. What are the major trends in the South Korea Point of Care Sepsis Diagnostics market?Key trends include Early diagnosis focus, biomarker adoption, and AI-enabled diagnostic platforms.
-
6. Who are the key companies operating in the South Korea Point of Care Sepsis Diagnostics market?Major players include Boditech Med Inc., SD Biosensor, Inc., Seegene, Inc., NanoEntek & Sugentech, Inc, Humasis CO., Ltd., Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, etc
-
7. What are the restraints affecting the South Korea Point of Care Sepsis Diagnostics market?High compliance costs for low-sulfur fuels, fluctuating crude oil prices, and infrastructure limitations in some ports restrain market growth.
-
8. What is the future outlook for the South Korea Point of Care Sepsis Diagnostics market?The market is expected to grow steadily, reaching USD 37.6 million by 2035 at a CAGR of 10.2%.
Need help to buy this report?