Singapore Clinical Trials Management System Market Size, Share, and COVID-19 Impact Analysis, By Delivery Mode (Web & Cloud-Based and On-Premise), By Component (Software and Services), By Solution Type (Enterprise and Site), and Singapore Clinical Trials Management System Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareSingapore Clinical Trials Management System Market Insights Forecasts to 2035
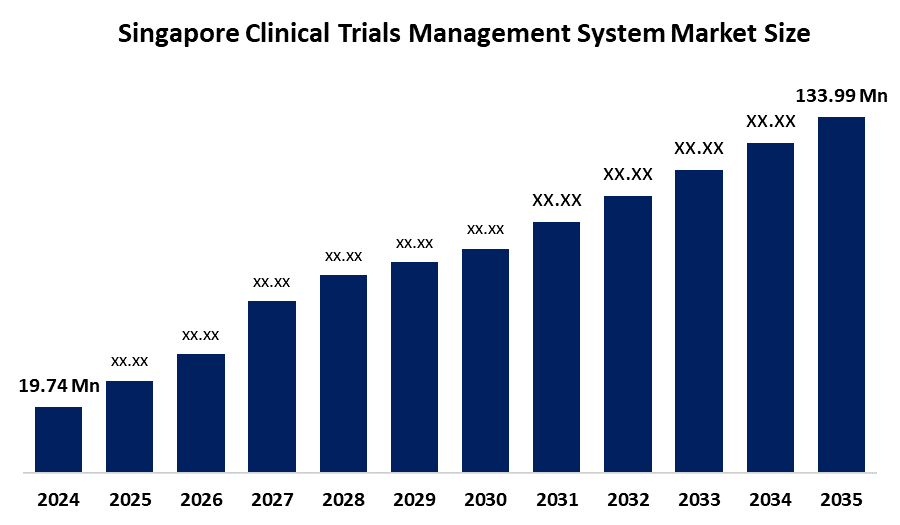
- The Singapore Clinical Trials Management System Market Size was estimated at USD 19.74 Million in 2024
- The Singapore Clinical Trials Management System Market Size is expected to grow at a CAGR of around 19.02% from 2025 to 2035
- The Singapore Clinical Trials Management System Market Size is expected to reach USD 133.99 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights and Consulting, the Singapore clinical trials management system market size is anticipated to reach USD 133.99 million by 2035, growing at a CAGR of 19.02 percent from 2025 to 2035. The Singapore clinical trials management system market is driven by a growing pharmaceutical and biotech industry, strong government support through agencies such as HSA and NMRC, advanced healthcare infrastructure, a skilled workforce, rising demand for new therapies, and increasing adoption of digital trial management systems.
Market Overview
The Singapore clinical trials management system (CTMS) market refers to the segment focused on software platforms and solutions that streamline the planning, execution, and monitoring of clinical trials in Singapore. CTMS solutions help manage study protocols, patient recruitment, site management, regulatory compliance, data collection, and reporting for pharmaceutical, biotechnology, and medical device trials. By improving efficiency, transparency, and data accuracy, CTMS platforms enable hospitals, research institutions, and CROs in Singapore to conduct trials faster, cost effectively, and in compliance with global regulatory standards, supporting the countrys position as a leading hub for clinical research.
The Singapore clinical trials management system market is increasingly important due to the countrys strong role as a clinical research hub and rising demand for efficient trial operations. Singapore has initiated around 1,179 clinical trials from 2020 to 2025, averaging about 220 trials annually, with nearly half sponsored by academia and hospitals and the rest by industry, highlighting robust research activity across phases I to III. The nation also averages 225 clinical trials per year, including 95 drug trials, underscoring significant demand for systems that streamline study design, patient recruitment, compliance, monitoring, and data management.
Government support further strengthens market adoption. The National Medical Research Council (NMRC) offers clinical trial grants up to SGD 4.94 million per project under the CTG ICT scheme and SGD 1.625 million per project under the CTG IIT scheme. Singapore also launched the ClinicalTrials.SG portal to centralize trial information and improve access for patients, clinicians, and sponsors, enhancing trial awareness and participation. These initiatives, combined with Singapores advanced healthcare infrastructure, rigorous regulatory standards, and a growing biomedical ecosystem, drive the need and importance of clinical trial management systems in the country.
Report Coverage
This research report categorizes the market for the Singapore clinical trials management system market based on various segments and regions, and forecasts revenue growth and analyzes trends in each submarket. The report analyzes the key growth drivers, opportunities, and challenges influencing the Singapore clinical trials management system market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition, have been included to illustrate the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyzes their core competencies in each sub segment of the Singapore clinical trials management system market.
Driving Factors
The Singapore clinical trials management system (CTMS) market is driven by growing clinical research activity, an expanding pharmaceutical and biotech sector, and advanced healthcare infrastructure. Strong government support with grants up to SGD 4.94 million, regulatory compliance requirements, and increasing adoption of digital trial management tools further boost demand and market growth.
Restraining Factors
The Singapore clinical trials management system (CTMS) market faces restraining factors such as high implementation and maintenance costs, complex integration with existing hospital and research IT systems, data privacy and cybersecurity concerns, and limited skilled personnel to manage and operate advanced CTMS platforms. Additionally, regulatory and compliance complexities can slow adoption, especially for smaller research institutions or startups.
Market Segmentation
The Singapore clinical trials management system market share is classified into delivery mode, component, and solution type.
- The web and cloud based segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Singapore clinical trials management system market is segmented by delivery mode into web and cloud based and on premise. Among these, the web and cloud based segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The web and cloud based segment is growing because it offers flexibility, remote accessibility, real time data sharing, and lower IT infrastructure costs compared to on premise systems. Its scalability, ease of updates, and support for multi site clinical trials make it ideal for Singapores growing number of cross institutional and global research studies, driving strong adoption.
- The software segment dominated the market in 2024 and is anticipated to grow at a substantial CAGR during the forecast period.
The Singapore clinical trials management system market is segmented by component into software and services. Among these, the software segment dominated the market in 2024 and is anticipated to grow at a substantial CAGR during the forecast period. The software segment is growing because it provides centralized management of clinical trial data, patient recruitment, protocol tracking, and regulatory compliance, improving efficiency and accuracy. Increasing adoption of digital tools and analytics by hospitals, CROs, and research institutions in Singapore drives strong demand for clinical trial management software.
- The enterprise segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Singapore clinical trials management system market is segmented by solution type into enterprise and site. Among these, the enterprise segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The enterprise segment is growing due to its ability to manage multiple clinical trials across various sites, provide centralized data management, and ensure regulatory compliance for large pharmaceutical companies and research organizations. Its scalability and integration capabilities make it ideal for Singapores expanding multicenter and multinational clinical studies, driving strong adoption.
Competitive Analysis:
The report offers an appropriate analysis of the key organizations and companies involved within the Singapore clinical trials management system market, along with a comparative evaluation primarily based on their product offerings, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on current news and developments of the companies, including product development, innovations, joint ventures, partnerships, mergers and acquisitions, strategic alliances, and other activities. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Oracle
- Medidata Solutions
- Parexel
- Bioclinica
- Bio Optronics
- IBM
- DATATRAK International
- Veeva Systems
- DSG
- MasterControl
- Clario
- IQVIA Holdings Inc.
- Others
Key Target Audience
- Market Players
- Investors
- End users
- Government Authorities
- Consulting and Research Firms
- Venture Capitalists
- Value Added Resellers (VARs)
Recent Developments
-
In July 2024, Singapores Clinical Research Institute (SCRI) launched ClinicalTrials.SG, a centralized portal to make clinical trial information more accessible for patients, caregivers, and researchers. This initiative strengthens Singapores clinical trials management ecosystem and boosts research participation.
Market Segment
This study forecasts revenue at the Singapore, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Singapore clinical trials management system market based on the below mentioned segments.
Singapore Clinical Trials Management System Market, By Delivery Mode
- Web and Cloud Based
- On Premise
Singapore Clinical Trials Management System Market, By Component
- Software
- Services
Singapore Clinical Trials Management System Market, By Solution Type
- Enterprise
- Site
Frequently Asked Questions (FAQ)
-
1.What is the Singapore clinical trials management system market size in 2024?The Singapore clinical trials management system market size was estimated at USD 19.74 million in 2024.
-
2.What is the projected market size of the Singapore clinical trials management system market by 2035?The Singapore clinical trials management system market size is expected to reach USD 133.99 million by 2035.
-
3.What is the CAGR of the Singapore clinical trials management system market?The Singapore clinical trials management system market size is expected to grow at a CAGR of around 19.02% from 2024 to 2035.
-
4.What are the key growth drivers of the Singapore clinical trials management system market?The Singapore clinical trials management system market is driven by a growing pharmaceutical and biotech industry, strong government support through agencies like HSA and NMRC, advanced healthcare infrastructure, a skilled workforce, rising demand for new therapies, and increasing adoption of digital trial management systems.
-
5.Which component segment dominated the market in 2024?The software segment dominated the market in 2024.
-
6.What segments are covered in the Singapore clinical trials management system market report?The Singapore clinical trials management system market is segmented on the basis of delivery mode, component, and solution type.
-
7.Who are the key players in the Singapore clinical trials management system market?Key companies include Oracle, Medidata Solutions, Parexel, Bioclinica, Bio‑Optronics, IBM, DATATRAK International, Veeva Systems, DSG, MasterControl, Clario, IQVIA Holdings Inc., and others.
-
8.Who are the target audiences for this market report?The report targets market players, investors, end-users, government authorities, consulting and research firms, venture capitalists, and value-added resellers (VARs).
Need help to buy this report?