Global Respiratory Antiviral Treatment Market Size, Share, and COVID-19 Impact Analysis, By Drug Class (Nucleoside Analogs, Neuraminidase Inhibitors, Ion Channel Blockers, and Fusion Protein Inhibitors), By Disease Type (Pneumonia, Influenza, Bronchiolitis, Upper Respiratory Tract Infection, and Others), By Distribution Channel (Hospital Pharmacy, Retail Pharmacy, and Online Pharmacy), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 - 2035
Industry: HealthcareGlobal Respiratory Antiviral Treatment Market Insights Forecasts To 2035
- The Global Respiratory Antiviral Treatment Market Size Was Estimated at USD 2.45 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of around 7.73% from 2025 to 2035
- The Worldwide Respiratory Antiviral Treatment Market Size is Expected to Reach USD 5.56 Billion by 2035
- Asia Pacific is expected to grow the fastest during the forecast period.
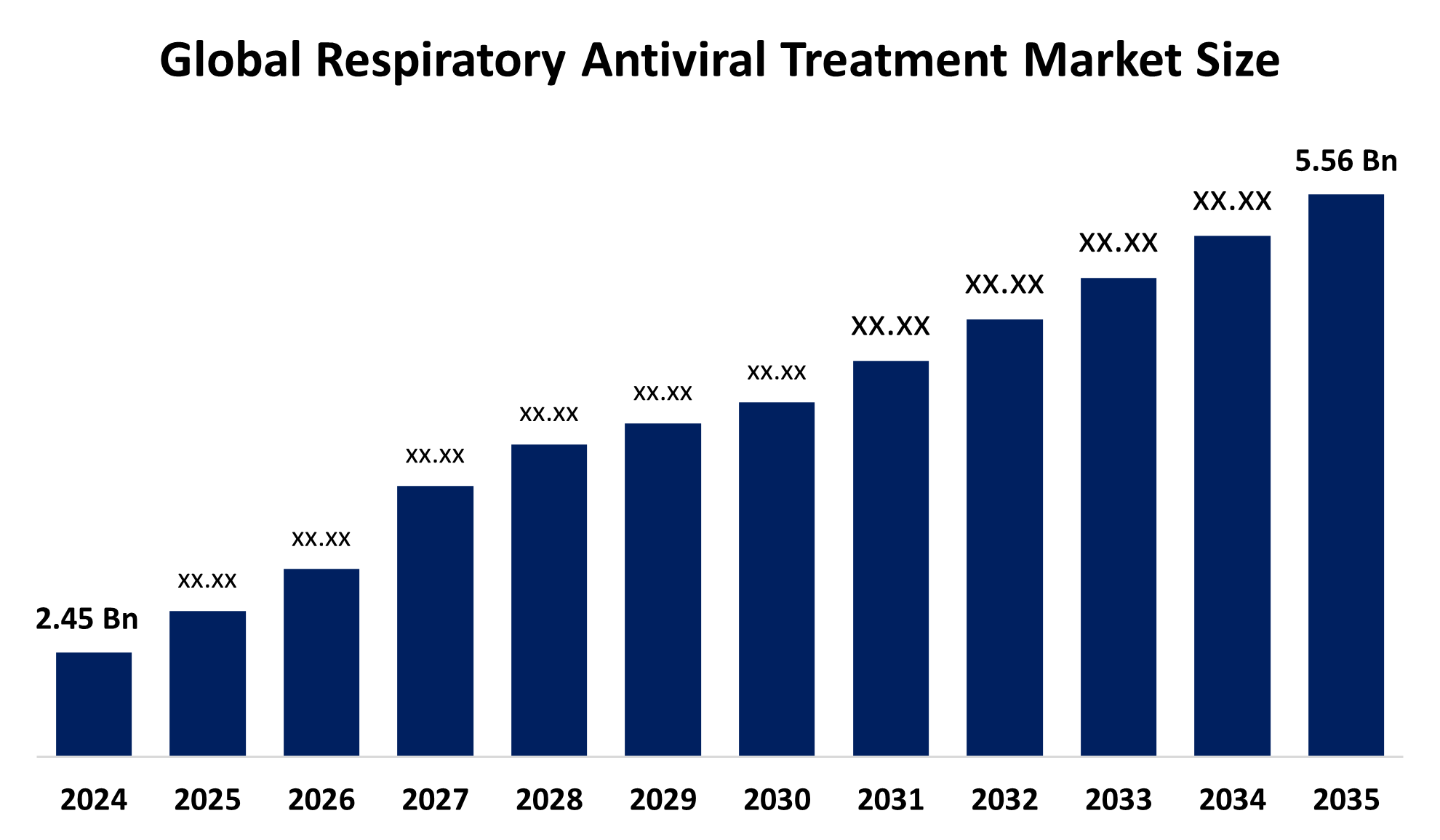
Get more details on this report -
According to a research report published by Spherical Insights and Consulting, The Global Respiratory Antiviral Treatment Market Size was worth around USD 2.45 Billion in 2024 and is Predicted to Grow to around USD 5.56 Billion by 2035 with a compound annual growth rate (CAGR) of 7.73% from 2025 and 2035. The market for respiratory antiviral treatment has a number of opportunities to grow due to the integration of new approaches in drug development.
Market Overview
The global industry of respiratory antiviral treatment refers to the development, manufacturing, and selling of drugs used for treating viral infections of the respiratory tract. Respiratory antiviral treatment involves the use of antiviral medications that are targeted at viruses, especially influenza. Further, the emergence of new pathogens like MERS-CoV is driving the need for developing new therapeutics, including clinical study examination of novel targets, combinations designed to increase potency & reduce resistance emergence, therapeutic antibodies, and immunomodulatory agents for mitigating immunopathologic host responses, especially for influenza. Further, there is ongoing development of antiviral drugs for managing bronchiolitis in young infants and newborns.
Innovation and market expansion are anticipated as a result of major players' growing R&D expenditures and the expanding partnerships. Further, there is increased investment by the private organization in supporting the clinical development of novel antivirals, which is promoting the respiratory antiviral treatment market. For instance, in February 2025, RNA Respiratory received US$5M investment from Flu Lab to support clinical development of novel intranasal antiviral host defence immune enhancer, INNA-051.
Report Coverage
This research report categorizes the respiratory antiviral treatment market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the respiratory antiviral treatment market. Recent market developments and competitive strategies such as expansion, type launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the respiratory antiviral treatment market.
Global Respiratory Antiviral Treatment Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 2.45 Billion |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 7.73% |
| 2035 Value Projection: | USD 5.56 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 235 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Drug Class, By Disease Type, By Distribution Channel, By Region |
| Companies covered:: | F. Hoffmann La Roche Ltd., Novartis AG, Mylan N.V., Sanofi, Pfizer Inc., GlaxoSmithKline plc, Merck & Co., Inc., Teva Pharmaceutical Industries Ltd., Dr. Reddy’s Laboratories Ltd., Zydus Cadila, Johnson & Johnson Private Limited, Amneal Pharmaceuticals LLC, AbbVie Inc., Alembic Pharmaceuticals Limited, Lupin, and Others |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The increased prevalence of viral respiratory diseases, is anticipated to drive the market demand. According to data published by the WHO, there are around a billion cases of seasonal influenza annually, including 3-5 million cases of severe illness, and causing 290 000 to 650 000 respiratory deaths annually. Increasing advancements in the development of antiviral strategies against pathogens, including the respiratory syncytial virus and SARS-CoV-2, are propelling market growth. Further, an increasing investment by organization in supporting R&D activities is promoting market growth. For instance, in January 2025, the U.S. Department of Health and Human Services (HHS) awarded $306 million to continue its H5N1 Avian Flu response.
Restraining Factors
The respiratory antiviral treatment market is restricted by factors like the increased cost of drugs and strict regulatory approval policies. Further, the potential of these drugs to cause antiviral resistance is challenging the market.
Market Segmentation
The respiratory antiviral treatment market share is classified into drug class, disease type, and distribution channel.
- The neuraminidase inhibitors segment held the largest market share of 54.3 % in 2024 and is projected to grow at a substantial CAGR during the forecast period.
Based on the drug class, the respiratory antiviral treatment market is divided into nucleoside analogs, neuraminidase inhibitors, ion channel blockers, and fusion protein inhibitors. Among these, the neuraminidase inhibitors segment held the largest market share of 54.3 % in 2024 and is projected to grow at a substantial CAGR during the forecast period. Neuraminidase inhibitors, like Zanamivir and Oseltamivir, aid in preventing the spread of viruses like influenza A and B. With an increasing advancement in medical science, the growing investment in R&D activities of antiviral drugs in the biopharma sectors is driving the market.
- The influenza segment dominated the respiratory antiviral treatment market with around 34.54 % share in 2024 and is anticipated to grow at a significant CAGR during the forecast period.
Based on the disease type, the respiratory antiviral treatment market is divided into pneumonia, influenza, bronchiolitis, upper respiratory tract infection, and others. Among these, the influenza segment dominated the respiratory antiviral treatment market with around 34.54 % share in 2024 and is anticipated to grow at a significant CAGR during the forecast period. As per the WHO, there are around a billion cases of seasonal influenza annually, causing 290,000 to 650,000 respiratory deaths annually. The growing prevalence of influenza is responsible for driving the market demand.
- The hospital pharmacy segment dominated the market with the largest revenue share of around 36-56% in 2024 and is anticipated to grow at a significant CAGR during the forecast period.
Based on the distribution channel, the respiratory antiviral treatment market is divided into hospital pharmacy, retail pharmacy, and online pharmacy. Among these, the hospital pharmacy segment dominated the market with the largest revenue share of around 36-56% in 2024 and is anticipated to grow at a significant CAGR during the forecast period. Hospital pharmacy supports pharmaceutical care services by collaborating with the multidisciplinary team. Increasing developments in hospital infrastructure, with government investments for developing integrated pharmacy hospitals, are propelling the market growth.
Regional Segment Analysis of the Respiratory Antiviral Treatment Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
North America is anticipated to hold the largest share of the respiratory antiviral treatment market over the predicted timeframe.
North America is anticipated to hold the largest share of around 37% in the respiratory antiviral treatment market over the predicted timeframe. The market ecosystem in North America is strong, due to the presence of leading companies like Pfizer, Merck, Gilead Sciences, and GSK, focusing on research and development of novel therapies for respiratory viruses. For instance, in October 2021, Merck plan to seek emergency use authorization in the U.S. and to submit applications to Regulatory Agencies Worldwide for molnupiravir. Government support for strengthening the healthcare sector in countries like the US, Canada, and Mexico is contributing to propelling the regional market. The United States held the dominant share of 74.87% in the North America respiratory antiviral treatment market, owing to the support of organisations like NIH for innovative research in biotech-based companies.
Asia Pacific is expected to grow at a rapid CAGR of 9.3% in the respiratory antiviral treatment market during the forecast period. The Asia Pacific area has a thriving market for respiratory antiviral treatment due to the growing prevalence of pneumonia, especially among children, along with an increasing investment in the development of the antiviral drug sector. China is leading the market in the APAC region, with about 42% regional market share, which is driven by the government support for combating viral infections and increasing antiviral drug approval. Furthermore, strategic alliances for enhancing the development of innovative therapies are supporting regional market growth. For instance, in May 2023, WuXi STA, and Shanghai Ark Biopharmaceutical Co., Ltd. (ArkBio) announced a strategic partnership for the commercial supply of Ziresovir, a novel treatment of respiratory syncytial virus (RSV) infections.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the respiratory antiviral treatment market, along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- F. Hoffmann La Roche Ltd.
- Novartis AG
- Mylan N.V.
- Sanofi
- Pfizer Inc.
- GlaxoSmithKline plc
- Merck & Co., Inc.
- Teva Pharmaceutical Industries Ltd.
- Dr. Reddy's Laboratories Ltd.
- Zydus Cadila
- Johnson & Johnson Private Limited
- Amneal Pharmaceuticals LLC
- AbbVie Inc.
- Alembic Pharmaceuticals Limited
- Lupin
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In September 2025, Enanta Pharmaceuticals would present top-line results from its Phase 2b Study Evaluating Zelicapavir for the Treatment of Respiratory Syncytial Virus (RSV) in High-Risk Adults.
- In September 2024, ArkBio Announces Publication of Phase 3 Clinical Trial Results of Ziresovir for the Treatment of RSV Infection in The New England Journal of Medicine.
- In January 2024, INTREPID Alliance Releases Review of Antiviral Compounds in Clinical Development to Contribute to Collaborative Efforts in Pandemic Preparedness.
- In February 2021, GlaxoSmithKline plc and Vir Biotechnology, Inc., announced they hhadsigned a binding agreement to expand their existing collaboration to include the research and development of new therapies for influenza and other respiratory viruses.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the respiratory antiviral treatment market based on the below-mentioned segments:
Global Respiratory Antiviral Treatment Market, By Drug Class
- Nucleoside Analogs
- Neuraminidase Inhibitors
- Ion Channel Blockers
- Fusion Protein Inhibitors
Global Respiratory Antiviral Treatment Market, By Disease Type
- Pneumonia
- Influenza
- Bronchiolitis
- Upper Respiratory Tract Infection
- Others
Global Respiratory Antiviral Treatment Market, By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
Global Respiratory Antiviral Treatment Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the respiratory antiviral treatment market over the forecast period?The global respiratory antiviral treatment market is projected to expand at a CAGR of 7.73% during the forecast period.
-
2. What is the market size of the respiratory antiviral treatment market?The global respiratory antiviral treatment market size is expected to grow from USD 2.45 Billion in 2024 to USD 5.56 Billion by 2035, at a CAGR of 7.73% during the forecast period 2025-2035.
-
3. Which region holds the largest share of the respiratory antiviral treatment market?North America is anticipated to hold the largest share of the respiratory antiviral treatment market over the predicted timeframe.
-
4. Who are the top companies operating in the Global Respiratory Antiviral Treatment Market?Key players include F. Hoffmann-La Roche Ltd., Novartis AG, Mylan N.V., Sanofi, Pfizer Inc., GlaxoSmithKline plc, Merck & Co., Inc., Teva Pharmaceutical Industries Ltd., Dr. Reddy's Laboratories Ltd., Zydus Cadila, Johnson & Johnson Private Limited, Amneal Pharmaceuticals LLC, AbbVie Inc., Alembic Pharmaceuticals Limited, and Lupin.
-
5. Can you provide company profiles for the leading respiratory antiviral treatment manufacturers?Yes. For example, F. Hoffmann-La Roche Ltd., a subsidiary of Roche Holding AG, is a biotechnology company and a provider of in-vitro diagnostics as well as a supplier of transformative innovative solutions across major disease areas. Novartis AG is a pharmaceutical company that is engaged in the research, development, manufacturing, distribution, marketing, and sale of innovative medicines.
-
6. What are the main drivers of growth in the respiratory antiviral treatment market?The growing prevalence of viral respiratory diseases, increasing R&D efforts, and advancement in drug development are major market growth drivers of the respiratory antiviral treatment market.
-
7. What challenges are limiting the respiratory antiviral treatment market?The potential of these drugs to cause antiviral resistance, high cost, and strict regulations are key restraints in the respiratory antiviral treatment market.
Need help to buy this report?