Brazil Pharmacovigilance and Drug Safety Software Market Size, Share, By Delivery Mode (On Premise and On Demand), By End Use (Healthcare Companies, CROs/BPOs/PV Service Providers, and Others), and Brazil Pharmacovigilance and Drug Safety Software Market Insights, Industry Trend, Forecasts to 2035
Industry: Information & TechnologyBrazil Pharmacovigilance and Drug Safety Software Market Insights Forecasts to 2035
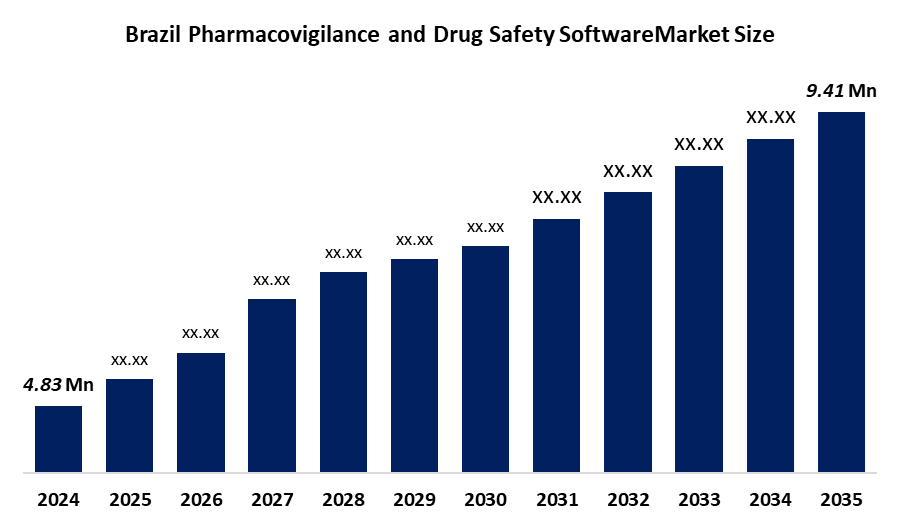
- Brazil Pharmacovigilance and Drug Safety Software Market Size 2024: USD 4.83 Mn
- Brazil Pharmacovigilance and Drug Safety Software Market Size 2035: USD 9.41 Mn
- Brazil Pharmacovigilance and Drug Safety Software Market CAGR 2024: 6.25%
- Brazil Pharmacovigilance and Drug Safety Software
Get more details on this report -
-
The market for Pharmacovigilance and Drug Safety Software encompasses all digital tools that allow organizations working in pharmaceuticals, biotechnology, and healthcare to ensure their products are safe by tracking, identifying, evaluating, and mitigating any harmful effects or potential safety issues that may arise from usage. The growth of the market is backed by the increased drug approval rates, more rigorous regulations for adverse event reporting and the adoption of cloud-based drug safety systems because of the ongoing digital transformation of the drug industry
- As technology advances, the Brazilian pharmacovigilance and drug safety software market has begun its transition to include AI and cloud-based Platforms and automation. To enhance the efficiency of the detection of adverse events and regulatory compliance through increased efficiencies in reporting, utilization of predictive analytics and real time safety monitoring to deal with complex safety data has improved the signal detection accuracy to enhance quality and faster decision making.
-
Market Dynamics of the Brazil Pharmacovigilance and Drug Safety Software Market:
The Brazil pharmacovigilance and drug safety software market is driven by the increase in reporting of ADRs, stricter requirements for regulatory compliance with ANVISA, and growing numbers of clinical trials. Automated, cloud-based pharmacovigilance solutions are currently very popular throughout Brazil, due in part to the growing number of digital health system providers and a growing need for real-time safety monitoring by the pharmaceutical industry, as well as an increase in post-marketing surveillance by pharmaceutical and biotech companies.
-
The Brazil pharmacovigilance and drug safety software market is restrained by the various factors such as high implementation and ongoing maintenance costs, lack of IT resources, limited availability of skilled personnel, difficulties in integrating new data into existing legacy systems, concerns about data privacy and data protection legislation, and controlling drug product for unauthorized use. Due to these limitations, digital adoption among smaller drug manufacturers has lagged larger drug manufacturers.
-
The Brazil pharmacovigilance and drug safety software market is well-positioned to benefit from evolving safety regulations enforced by ANVISA, increased numbers of clinical trials being conducted in the country, and the continued development of domestic pharmaceutical manufacturing operations. Furthermore, the adoption of artificial intelligence, cloud-based platforms, analytics based on real-world evidence, and outsourced pharmacovigilance operations will create additional growth opportunities throughout the country.
- Market Segments: Delivery mode and End Use
Brazil Pharmacovigilance and Drug Safety Software Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 4.83 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR of 6.25% |
| 2035 Value Projection: | USD 9.41 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 210 |
| Tables, Charts & Figures: | 90 |
| Segments covered: | By Delivery, By End |
| Companies covered:: | ArisGlobal, IQVIA Holdings Inc, Capgemini SE, PAREXEL, Icon PLC, Cognizant Technology Solutions Corp Class A, Labcorp Holdings Inc, and other key players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Market Segmentation
The Brazil Pharmacovigilance and Drug Safety Software Market share is classified into delivery mode and end use.
By Delivery Mode:
The Brazil pharmacovigilance and drug safety software market is divided by delivery mode into on premise and on demand. Among these, the on-demand segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. Brazil has the largest segment of the on-demand segment because of its low initial investment, scalability, speed of implementation, automatic reporting of safety in real time, automatic updates to regulatory compliance, and remote access. These advantages are beneficial to both pharmaceutical companies and CROs that are looking for a flexible, compliant, and cost-effective solution for pharmacovigilance.
By End Use:
The Brazil pharmacovigilance and drug safety software market is divided by end use into healthcare companies, CROs/BPOs/PV service providers, and others. Among these, the healthcare companies segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. The healthcare companies segment dominates because it represents the highest volume of drug safety data being processed by pharmaceutical manufacturers. Because pharmaceutical manufacturers are required by law to report adverse events, ensure regulatory compliance, and manage risk, they must continue to use pharmacovigilance software as an integral part of their operations on an ongoing basis.
Competitive Analysis:
The report offers the appropriate analysis of the key organisations/companies involved within the Brazil pharmacovigilance and drug safety software market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
Top Key Companies in Brazil Pharmacovigilance and Drug Safety Software Market:
- ArisGlobal
- IQVIA Holdings Inc
- Capgemini SE
- PAREXEL
- Icon PLC
- Cognizant Technology Solutions Corp Class A
- Labcorp Holdings Inc
- Other
Recent Developments in Brazil Pharmacovigilance and Drug Safety Software Market:
In September 2025, ANVISA reinforced its commitment to drug safety at the XX International Pharmacovigilance Meeting of the Americas, where it participated alongside regulators from over 20 countries to discuss active vaccine pharmacovigilance, AI-enabled safety monitoring, international cooperation, and signal management strategies, thereby strengthening Brazil’s post-market surveillance system and alignment with global regulatory standards.
In April 2025, ANVISA held the 2nd International Pharmacovigilance Symposium in Brasília, which brought together regulators, technical committees, academia, and international partners to share insights on adverse event reporting workflows, challenge areas, and collaborative methods aimed at improving patient safety across Brazil’s pharmacovigilance ecosystem.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the Brazil regional, and country levels from 2020 to 2035. Decisions Advisors has segmented the Brazil pharmacovigilance and drug safety software market based on the below-mentioned segments:
Brazil Pharmacovigilance and Drug Safety Software Market, By Delivery Mode
- On Premise
- On Demand
Japan Brazil Pharmacovigilance and Drug Safety Software Market, By End Use
- Healthcare Companies
- CROs/BPOs/PV service providers
- Others
Frequently Asked Questions (FAQ)
-
What is the Brazil pharmacovigilance and drug safety software market size?Brazil Pharmacovigilance and Drug Safety Software Market is expected to grow from USD 4.83 million in 2024 to USD 9.41 million by 2035, growing at a CAGR of 6.25% during the forecast period 2025-2035.
-
How is the market segmented by delivery mode type?The market is segmented into on premise and on demand.
-
Who are the key players in the Brazil pharmacovigilance and drug safety software market?Key companies include ArisGlobal, IQVIA Holdings Inc, Capgemini SE, PAREXEL, Icon PLC, Cognizant Technology Solutions Corp Class A, Labcorp Holdings Inc, and Other.
-
Who are the target audiences for this market report?The report targets market players, investors, end-users, government authorities, consulting and research firms, venture capitalists, and value-added resellers (VARs).
Need help to buy this report?