Global Pharmaceutical Intellectual Property and Generics Market Size, Share, and COVID-19 Impact Analysis, By Drug Type (Branded Drugs and Generic Drugs), By Therapeutic Area (Cardiovascular, Oncology, Neurology, Infectious Diseases, and Others), and by Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 - 2035
Industry: HealthcareGlobal Pharmaceutical Intellectual Property and Generics Market Insights Forecasts to 2035
- The Market Size is Expected to Grow at a CAGR of around 6.3% from 2025 to 2035
- The Global Pharmaceutical Intellectual Property and Generics market size is expected to hold a significant share by 2035,
- Asia Pacific is Expected to Grow the fastest during the forecast period.
Get more details on this report -
According to a research report published by Spherical Insights and Consulting, The Global Pharmaceutical Intellectual Property And Generics Market Size is expected to hold a significant share by 2035, at a CAGR of 6.3% during the forecast period 2025-2035. The pharmaceutical intellectual property and generics market offers opportunities such as using patent expirations to develop biosimilars, streamlining drug innovation with AI, and satisfying the growing demand for affordable generics worldwide in the face of rising healthcare demands and regulatory support.
Global Pharmaceutical Intellectual Property and Generics Market Forecast and Revenue Outlook
- CAGR (2025-2035): 6.3%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
Market Overview
The legal rights that safeguard innovations in the pharmaceutical sector, such as patents, trademarks, and copyrights for new medications, formulations, and production techniques, are referred to as pharmaceutical intellectual property and generics market. Pharmaceutical products that are bioequivalent to branded medications and are released after patents have expired constitute a rise in the generics market. Generics provide affordable substitutes that improve healthcare affordability and accessibility while upholding safety and efficacy regulations. The FDA's Generic Drug User Fee Amendments (GDUFA), which expedited the entry of generic drugs into the market by facilitating 250 ANDA approvals between October 2024 and February 2025, is one of the strong U.S. government initiatives supporting the pharmaceutical intellectual property (IP) and generics market. Economic, technological, regulatory, and sociological elements that encourage innovation and support access to inexpensive healthcare are the driving forces behind the pharmaceutical intellectual property and generics market. Generics are in high demand due to growing healthcare demands worldwide, which are driven by aging populations, the incidence of chronic diseases, and more access in emerging countries.
Key Market Insights
- North America is expected to account for the largest share of the pharmaceutical intellectual property and generics market during the forecast period.
- In terms of drug type, the generic drugs segment is projected to lead the pharmaceutical intellectual property and generics market throughout the forecast period
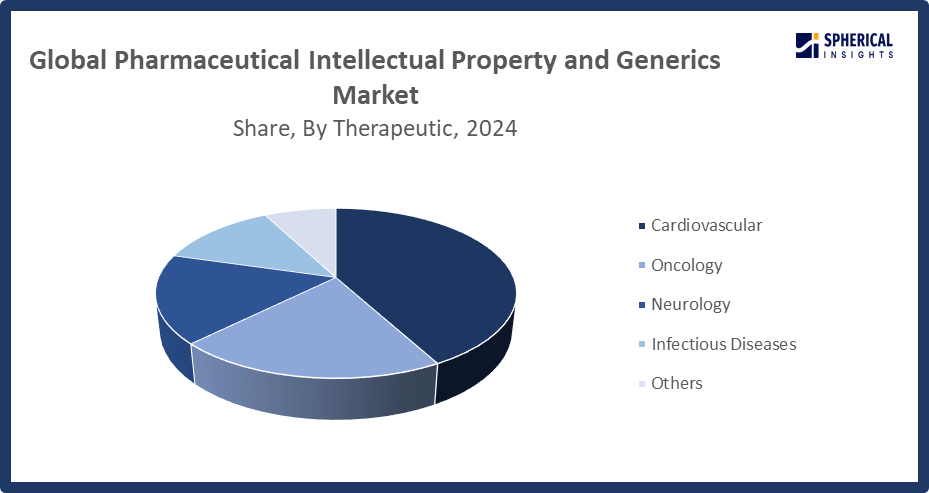
- In terms of therapeutic area, the cardiovascular segment captured the largest portion of the market
Pharmaceutical Intellectual Property and Generics Market Trends
- Countries are more frequently invoking TRIPS flexibilities for public health.
- Growing use of compulsory licensing in low- and middle-income countries.
- Digital health and AI-driven drug discovery are expanding IP domains.
- Biologics and biosimilars create complex IP and regulatory landscapes.
- Data exclusivity periods continue to delay generic market entry.
- Rise in patent litigation and challenges by generic manufacturers.
Report Coverage
This research report categorizes the pharmaceutical intellectual property and generics market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyzes the key growth drivers, opportunities, and challenges influencing the pharmaceutical intellectual property and generics market. Recent market developments and competitive strategies, such as expansion, type launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyzes their core competencies in each sub-segment of the pharmaceutical intellectual property and generics market.
Global Pharmaceutical Intellectual Property and Generics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 6.3% |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 217 |
| Tables, Charts & Figures: | 130 |
| Segments covered: | By Drug Type, By Therapeutic Area and by Region |
| Companies covered:: | Sandoz, Pfizer Inc., Bayer AG, Sanofi S.A., AbbVie Inc., Roche Group, Novartis AG, Merck & Co., Inc., Johnson & Johnson, Eli Lilly and Company, Teva Pharmaceuticals, Dr. Reddy’s Laboratories, Sun Pharmaceutical Industries Ltd., Cipla Ltd, and Others |
| Pitfalls & Challenges: | COVID-19 Impact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving factors
The patents on popular medications, such as rivaroxaban (Xarelto) and liraglutide (Victoza), are expiring, which creates room for biosimilars and generics, drastically lowering prices and driving the pharmaceutical intellectual property and generics market. The pharmaceutical IP and generics market is driven by a number of factors that improve affordability and innovation, including patent expirations, growing healthcare demand, AI-driven R&D efficiencies, supporting legislation as GDUFA, cost pressures, and strategic acquisitions. Global pharmaceutical intellectual property and generics market integration is also boosted by regulatory harmonization across regions, which speeds up generic approvals. These factors guarantee a strong balance between extending generics to effectively fulfill rising healthcare demands and safeguarding intellectual property to encourage innovation.
Restraining Factor
The pharmaceutical intellectual property and generics market is hampered by a number of problems, including tight patent litigation, complicated regulations, high R&D expenses, supply chain interruptions, and trade obstacles, including import bans from the United States to restrict market access and expansion.
Market Segmentation
The global pharmaceutical intellectual property and generics market is divided into drug type and therapeutic area.
Global Pharmaceutical Intellectual Property and Generics Market, By Drug Type:
- The generic drugs segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period.
Based on drug type, the global pharmaceutical intellectual property and generics market is segmented into branded drugs and generic drugs. Among these, the generic drugs segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period. The market for generic drugs is fueled by cost-effectiveness, patent expirations, and regulatory approvals, such as the FDA's anticipated 694 generic approvals. The use of generic drugs has also been accelerated by the rising incidence of chronic illnesses and rising healthcare costs in emerging nations.
The branded drugs segment in the pharmaceutical intellectual property and generics market is expected to grow at the fastest CAGR over the forecast period. The launch of novel treatments, consistent investment in R&D, and robust intellectual property rights that guarantee market exclusivity and encourage higher pricing schemes are the main drivers of the branded drug market.
Global Pharmaceutical Intellectual Property and Generics Market, By Therapeutic Area:
- The cardiovascular segment accounted for the largest share in 2024 and is anticipated to grow at a significant CAGR during the forecast period.
Based on therapeutic area, the global pharmaceutical intellectual property and generics market is segmented into cardiovascular, oncology, neurology, infectious diseases, and others. Among these, the cardiovascular segment accounted for the largest share in 2024 and is anticipated to grow at a significant CAGR during the forecast period. The increasing worldwide prevalence of cardiovascular disorders, such as heart failure, coronary artery disease, and hypertension, which still present serious public health issues, is the main cause of the cardiovascular section. Furthermore, several popular cardiovascular medications have seen their patents expire, which has made it easier for generic substitutes to enter the market and increase market.
Get more details on this report -
The oncology segment in the pharmaceutical intellectual property and generics market is expected to grow at the fastest CAGR over the forecast period. Growth in the oncology segment is driven by the rising prevalence of cancer worldwide, the growing need for affordable cancer therapies, and the expiration of patents on popular cancer medications, allowing it easier for generic and biosimilar alternatives to enter the market.
Regional Segment Analysis of the Global Pharmaceutical Intellectual Property and Generics Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
North America Pharmaceutical Intellectual Property and Generics Market Trends
North America is expected to hold the largest share of the global pharmaceutical intellectual property and generics market over the forecast period.
North America's market is guaranteed by its sophisticated healthcare system, high drug usage, and supportive regulations. The market for pharmaceuticals in the United States, which is expected to be worth USD 660 billion in 2025, benefits from widespread patent expirations that make generics and biosimilars more affordable. In order to meet public health needs, government pronouncements stress the importance of prioritizing generics first, but laws like the Hatch-Waxman Act strike a balance between competition and intellectual property protection.
United States Pharmaceutical Intellectual Property and Generics Market Trends
Intellectual property (IP) regimes that are strong and safeguard innovation through patents and regulatory exclusivities govern the pharmaceutical market in the United States. Generic competition is delayed by these procedures, which grant brand-name medication makers temporary market exclusivity. Generic medications become available on the market after patents expire, increasing competition and lowering medical expenses.
Asia Pacific Pharmaceutical Intellectual Property and Generics Market Trends
Asia Pacific is expected to grow at the fastest CAGR in the pharmaceutical intellectual property and generics market during the forecast period.
The Asia-Pacific region is experiencing a surge in healthcare demands due to factors such as growing access to healthcare in nations like China and India, large populations, and the prevalence of chronic diseases. Government programs like the Production Linked Incentive (PLI) Scheme and the Promotion of Research and Innovation in the Pharma-MedTech Sector (PRIP), which support the development of generic and biosimilar drugs, are helping to support India's pharmaceutical exports, which grew 7.8% in April 2025.
China Pharmaceutical Intellectual Property and Generics Market Trends
The pharmaceutical industry in China has experienced substantial changes to improve innovation and preserve intellectual property (IP), bringing it closer to international norms. Innovative pharmaceuticals are now better protected thanks to the introduction of patent linkage, data exclusivity, and a patent term extension scheme. Generics are guaranteed to meet quality and efficacy requirements equivalent to those of original medications, according to the generic drug consistency evaluation policy.
Europe Pharmaceutical Intellectual Property and Generics Market Trends
Europe is expected to hold a significant share of the global pharmaceutical intellectual property and generics market over the forecast period.
Europe is driven by robust intellectual property rights and a well-established legal framework. In order to maintain market integrity and public safety, the European Medicines Agency (EMA) and national regulatory agencies make sure that both novel and generic medications are rigorously evaluated. The affordability and accessibility of generic drugs are also improved by programs encouraging their adoption, such as reference pricing and substitution rules.
Germany Pharmaceutical Intellectual Property and Generics Market Trends
Strong intellectual property laws that promote innovation and patent exclusivity are a defining feature of the German pharmaceutical sector. The nation's regulatory structure encourages competition and cost containment by facilitating the introduction of high-quality generic medications through stringent clearance procedures. Reference pricing and reimbursement incentives are two examples of policies that promote the use of generic drugs while striking a balance between affordability and innovation.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global pharmaceutical intellectual property and generics market, along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
Worldwide Top Key Players in the Pharmaceutical Intellectual Property and Generics Market Include
- Sandoz
- Pfizer Inc.
- Bayer AG
- Sanofi S.A.
- AbbVie Inc.
- Roche Group
- Novartis AG
- Merck & Co., Inc.
- Johnson & Johnson
- Eli Lilly and Company
- Teva Pharmaceuticals
- Dr. Reddy’s Laboratories
- Sun Pharmaceutical Industries Ltd.
- Cipla Ltd
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent development
- In July 2025, the Association for Accessible Medicines provided feedback on a white paper titled "Long-Term Effects of Medicare Price Negotiations on Drug Competition," launched by Matrix Global Advisors. The paper examines the long-term effects on the development of new, less expensive medications, the impact and unintended consequences of the Medicare drug price negotiation program on generic and biosimilar medications, and the impact it will have on patient access to life-saving and cost-effective medications.
- In June 2025, Sun Pharmaceutical Industries Ltd. announced that it is ending clinical studies of its new medication candidate for atopic dermatitis and severe psoriasis because it failed to achieve its main goal. Wockhardt Ltd., another major pharmaceutical company based in Mumbai, announced about six months ago that its flagship discovery product, Zaynich, a novel antibiotic, had successfully finished a global, pivotal, registration-enabling clinical trial study and shown superiority over another medication that is regarded as the gold standard.
- In March 2025, The generic version and fixed dose combination (FDCs) of the diabetes medication empagliflozin can be introduced in India by Mankind Pharma (NSE: MANKIND), a Mumbai, India-based pharmaceutical company, at a 90% discount to the original product, Jardiance, made by Boehringer Ingelheim, according to Rajeev Juneja, vice chairman and managing director of the company.
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the pharmaceutical intellectual property and generics market based on the following segments:
Global Pharmaceutical Intellectual Property and Generics Market, By Drug Type
- Branded Drugs
- Generic Drugs
Global Pharmaceutical Intellectual Property and Generics Market, By Therapeutic Area
- Cardiovascular
- Oncology
- Neurology
- Infectious Diseases
- Others
Global Pharmaceutical Intellectual Property and Generics Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the pharmaceutical intellectual property and generics market over the forecast period?The global pharmaceutical intellectual property and generics market is projected to expand at a CAGR of 6.3% during the forecast period.
-
2. What is the market size of the pharmaceutical intellectual property and generics market?The global pharmaceutical intellectual property and generics market size is expected to hold a significant share by 2035, at a CAGR of 6.3% during the forecast period 2025-2035.
-
3. Which region holds the largest share of the pharmaceutical intellectual property and generics market?North America is anticipated to hold the largest share of the pharmaceutical intellectual property and generics market over the predicted timeframe.
-
4. Who are the top companies operating in the global pharmaceutical intellectual property and generics market?Sandoz, Pfizer Inc., Bayer AG, Sanofi S.A., AbbVie Inc., Roche Group, Novartis AG, Merck & Co., Inc., Johnson & Johnson, Eli Lilly and Company, Teva Pharmaceuticals, Dr. Reddy’s Laboratories, Sun Pharmaceutical Industries Ltd., Cipla Ltd, and others.
-
5. What factors are driving the growth of the pharmaceutical intellectual property and generics market?The growth of the pharmaceutical intellectual property and generics market is driven by rising demand for affordable medicines, patent expirations, supportive regulations, increasing chronic disease prevalence, and advancements in drug development technologies.
-
6. What are market trends in the pharmaceutical intellectual property and generics market?Increased use of biosimilars, improved patent protections, a rising emphasis on customized treatment, growth in new markets, and quicker regulatory approvals for generics are some of the major market trends in the pharmaceutical intellectual property and generics sector.
-
7. What are the main challenges restricting wider adoption of the pharmaceutical intellectual property and generics market?The main challenges restricting wider adoption include stringent regulatory requirements, patent litigations, pricing pressures, limited physician and patient awareness, and concerns regarding the quality and efficacy of generic medicines.
Need help to buy this report?