North America Tuberculosis Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Test Type (Detection of Latent Infection, Phage Assay, Detection of Drug Resistance, Nucleic Acid Testing, Radiographic Method, Cytokine Detection Assay, and Others), By End User (Diagnostic Laboratories, Hospitals & Clinics, and Others), and North America Tuberculosis Diagnostics Market Insights Forecasts to 2035
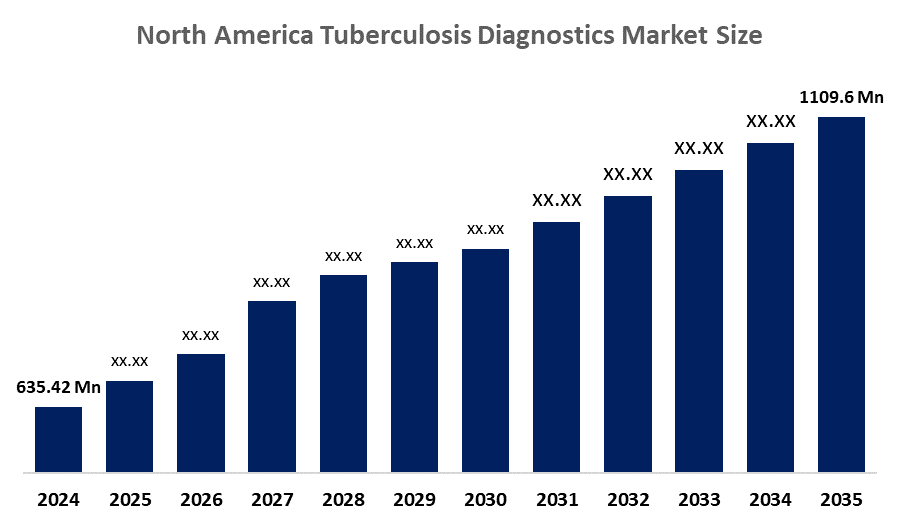
Industry: Healthcare- The North America Tuberculosis Diagnostics Market Size Was Estimated at USD 635.42 Million in 2024
- The Market Size is Expected to Grow at a CAGR of Around 5.2% from 2025 to 2035
- The North America Tuberculosis Diagnostics Market Size is Expected to Reach USD 1109.6 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the North America Tuberculosis Diagnostics Market size is anticipated to reach USD 1109.6 Million by 2035, growing at a CAGR of 5.2% from 2025 to 2035. The market is driven by advanced healthcare, strong government support, high awareness, and technological innovation.
Market Overview
Tuberculosis is a communicable disease that is brought about by Mycobacterium tuberculosis, a type of bacterium. The most common site infected is the lung of the patient. The infection may also invade the brain and the spine, among other body parts. Diagnosis of tuberculosis can be made by means of skin and blood tests. The rise in the number of cases of this condition has resulted in an increased demand for reliable and fast diagnostic tests to help in controlling the disease. It is estimated that only around 10% of latent infections turn into active disease, which in turn kills about half of the affected persons if no treatment is administered.
In the month of April 2025, Revvity obtained the FDA's blessing for its Auto-Pure 2400 platform that works alongside the T-SPOT.TB test, thereby making tuberculosis diagnostics automatic and more accurate. Revvity, Inc. announced in April 2025 that the Food and Drug Administration (FDA) of the United States had approved the Auto-Pure 2400 liquid handling platform with the T-SPOT.TB test.
The U.S. Centers for Disease Control and Prevention (CDC) has provided significant financial support to TB prevention and control initiatives. The Food and Drug Administration has taken back its final guidance on tuberculosis and sepsis for January 2025 and has reissued both as draft guidance with amendments derived from comments received.
Report Coverage
This research report categorizes the market for the North America tuberculosis diagnostics market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the North America tuberculosis diagnostics market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the North America tuberculosis diagnostics market.
North America Tuberculosis Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 635.42 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR of 5.2% |
| 2035 Value Projection: | USD 1109.6 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 250 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Test Type, By End User |
| Companies covered:: | Abbott Laboratories, Becton, Dickinson and Company, Cepheid, F. Hoffmann-La Roche AG, QIAGEN, Thermo Fisher Scientific, CTK Biotech, Biodesix, Teco Diagnostics, Tribal Diagnostics, and other key players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The tuberculosis diagnostics market in North America is driven by the region's high TB incidence is high and supported by appropriate health system policies. The World Economic Forum has pointed out that North America is a strong economy, responsible for 27.95% of global GDP, which weighs, among others, in the direction of the healthcare sector. The US healthcare costs are much higher than those of Canada. The need for TB diagnosing instruments and treatment is so great in both the public and private health sectors that it has become a major factor propelling this market forward.
Restraining Factors
The tuberculosis diagnostics market in North America is restrained in the U.S., advanced TB diagnosis tests, among which nucleic acid amplification tests (NAATs) and interferon gamma release assays (IGRA) are included, are frequently more costly than classical methods like sputum smear microscopy and culture-based TB diagnostics. Primary care centers catering to high-risk groups are commonly unable to secure the necessary funds to provide advanced diagnostics as part of their daily routine.
Market Segmentation
The North America tuberculosis diagnostics market share is categorised into test type and end user.
- The detection of latent infection segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The North America tuberculosis diagnostics market is segmented by test type into detection of latent infection, phage assay, detection of drug resistance, nucleic acid testing, radiographic method, cytokine detection assay, and others. Among these, the detection of latent infection segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. Detection of latent infection (skin test & IGRAs) was the largest segment with a revenue share of 41.26% in 2024. The growing use of the interferon-gamma release assays (IGRAs) and tuberculin skin tests (TSTs) for the critical need to identify asymptomatic carriers who later become active patients refers to their proven sensitivity and specificity. These tests are the main ones in the detection of Mycobacterium tuberculosis infections before symptoms occur.
- The diagnostic laboratories segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period.
Based on end user, the North America tuberculosis diagnostics market is segmented into diagnostic laboratories, hospitals & clinics, and others. Among these, the diagnostic laboratories segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period. The segmental growth is driven due to their unique skills and facilities; those laboratories can do that. Being able to control all the conditions, they can conduct the complex TB tests and get very precise and certain results. Moreover, one of the reasons healthcare workers highly prefer them is that they usually have both the best diagnostic equipment and the most professional staff available.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the North America tuberculosis diagnostics market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Abbott Laboratories
- Becton, Dickinson and Company
- Cepheid
- F. Hoffmann-La Roche AG
- QIAGEN
- Thermo Fisher Scientific
- CTK Biotech
- Biodesix
- Teco Diagnostics
- Tribal Diagnostics
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
In April 2025, Tulane University scientists created a groundbreaking handheld device that can diagnose tuberculosis (TB) in under an hour and with just a saliva sample. The device, called the lab-in-tube assay (LIT), offers a cost-effective, battery-powered solution ideal for rural and resource-limited areas.
In April 2024, Revvity, Inc. announced the launch of the Auto-Pure 2400 liquid handler from Allsheng for use with the T-SPOT.TB test. The Auto-Pure 2400 platform is easy to use and designed to provide efficient workflows in the lab.
Market Segment
This study forecasts revenue at the North America, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the North America Tuberculosis Diagnostics Market based on the below-mentioned segments:
North America Tuberculosis Diagnostics Market, By Test Type
- Detection of Latent Infection
- Phage Assay
- Detection of Drug Resistance
- Nucleic Acid Testing
- Radiographic Method
- Cytokine Detection Assay
- Others
North America Tuberculosis Diagnostics Market, By End User
- Diagnostic Laboratories
- Hospitals & Clinicas
- Others
Frequently Asked Questions (FAQ)
-
Q: What is the North America tuberculosis diagnostics market size?A: The North America Tuberculosis Diagnostics Market size is expected to grow from USD 635.42 million in 2024 to USD 1109.6 million by 2035, growing at a CAGR of 5.2% during the forecast period 2025-2035
-
Q: What is tuberculosis diagnostics, and its primary use?A: Tuberculosis is a communicable disease that is brought about by Mycobacterium tuberculosis, a type of bacterium. The most common site infected is the lung of the patient. The infection may also invade the brain and the spine, among other body parts. Diagnosis of tuberculosis can be made by means of skin and blood tests. The rise in the number of cases of this condition has resulted in an increased demand for reliable and fast diagnostic tests to help in controlling the disease
-
Q: What are the key growth drivers of the market?A: Market growth is driven by the region's high TB incidence is high and supported by appropriate health system policies. The World Economic Forum has pointed out that North America is a very powerful economy, responsible for 27.95% of global GDP, which weighs, among others, in the direction of the healthcare sector. In fact, the US healthcare costs are much higher than those of Canada
-
Q: What factors restrain the North America tuberculosis diagnostics market?A: The market is restrained by the fact that in the U.S., advanced TB diagnosis tests, among which nucleic acid amplification tests (NAATs) and interferon gamma release assays (IGRA) are included, are frequently more costly than classical methods like sputum smear microscopy and culture-based TB diagnostics
-
Q: How is the market segmented by test type?A: The market is segmented into detection of latent infection, phage assay, detection of drug resistance, nucleic acid testing, radiographic method, cytokine detection assay, and others)
-
Q: Who are the key players in the North America tuberculosis diagnostics market?A: Key companies include Abbott Laboratories, Becton, Dickinson and Company, Cepheid, F. Hoffmann-La Roche AG, QIAGEN, Thermo Fisher Scientific, CTK Biotech, Biodesix, Teco Diagnostics, and Tribal Diagnostics.
Need help to buy this report?