North America Guidewires Market Size, Share, and COVID-19 Impact Analysis, By Product (Surgical Guidewires and Diagnostic Guidewires), By Application (Coronary, Neurovascular, Urology, and Others), By End User (Hospitals, Diagnostic Centers and Surgical Cenetrs, Ambulatory Care Centers, and Others), and North America Guidewires Market Insights, Industry Trends, Forecast to 2035
Industry: HealthcareNorth America Guidewires Market Insights Forecasts to 2035
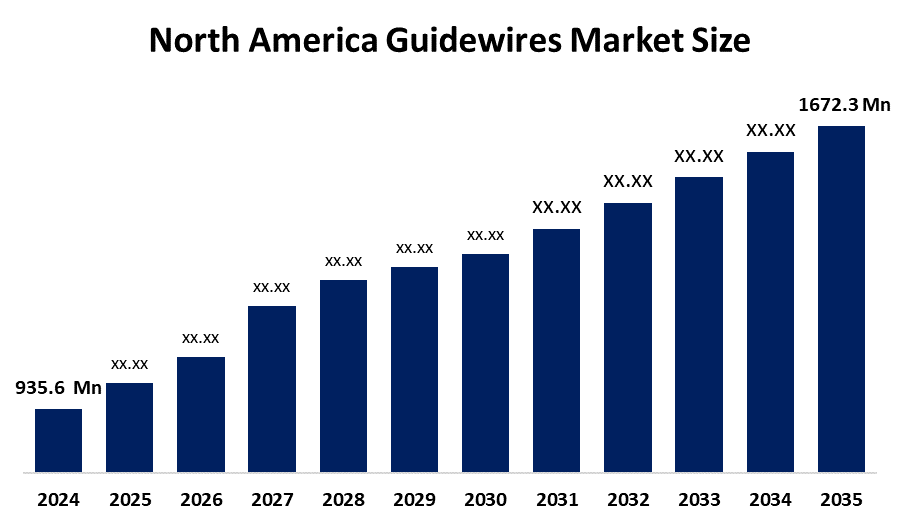
- The North America Guidewires Market Size Was Estimated at USD 935.6 Million in 2024
- The Market Size is Expected to Grow at a CAGR of Around 5.42% from 2025 to 2035
- The North America Guidewires Market Size is Expected to Reach USD 1672.3 Million by 2035
Get more details on this report -
According to a Research Report Published by Spherical Insights & Consulting, The North America Guidewires Market Size Is Anticipated To Reach USD 1672.3 Million By 2035, Growing At A CAGR Of 5.42% From 2025 To 2035. The market is driven by an elevated rate of chronic illnesses and the use of minimally invasive techniques, a medical device that safely passes catheters and other tools through the intricate anatomical systems of the body.
Market Overview
A guidewire is a slender, adaptable wire usually composed of nitinol or stainless steel, and often the flexible tip configuration and specialized coatis are applied to it. Purpose of the guidewire is to provide a safe temporary pathway during the accurate positioning and navigation of larger diagnostic or therapeutic medical devices to a lesion or area within the body, thus reducing the trauma to the surrounding tissues. It has been in extensive use in various medical interventions, including angioplasty, stenting, and management of coronary artery disease.
In June 2024, Medtronic introduced the steerant aortic guidewire, which was specifically designed to support catheter placement and exchange in the aorta during both diagnostic and interventional procedures. Guidewires play a key role in the aortic procedure, as they make it possible to follow the patient’s anatomy and bring the stent graft to the appropriate location.
Organizations are progressively implementing strategic measures to boost growth beyond the original predictions. The Food and Drug Administration (FDA) considers guidewires as Class II medical devices, which have to undergo a demanding premarket notification process. The medical reimbursement policies, particularly for procedures using guidewires, like angioplasty and stent placement, play an important role in attracting healthcare provider acceptance and are thus a major driving force of the market.
Report Coverage
This research report categorises the North America guidewires market based on various segments and regions, forecasting revenue growth and analysing trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the North America guidewires market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the North America guidewires market.
North America Guidewires Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 935.6 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 5.42% |
| 2035 Value Projection: | USD 1672.3 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 230 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Product, By Application, By End User |
| Companies covered:: | Boston Scientific Corporation, Medtronic PLC, Abbott Laboratories, Terumo Corporation, Stryker Corporation, Cook Medical, B. Braun Melsungen AG,, Cardinal Health, Teleflex Incorporated, ASAHI INTECC, and other key players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The guidewires market in North America is driven by the rising incidence of heart ailments, peripheral artery complications, and neurovascular disorders, which are forcing reliance on guidewire interventions. Among the intervention methods, guidewires are the least invasive, leading to quicker recovery and lower risk of infection, which, in turn, encourages their use. The development of less-invasive methods of taking nitinol alloys, combining hydrophilic/hydrophobic coatings and new designs, has kept improving guidewire flexibility, trackability, and safety and thus has improved the treatment results.
Restraining Factors
The guidewires market in North America is restrained by sophisticated guidewires are substantially costlier than traditional ones since they are composed of specific materials like hybrid polymers and nitinol. Getting regulatory authorisation from organisations like the U.S. FDA is an expensive and time-consuming process that requires a lot of clinical research and paperwork.
Market Segmentation
The North America guidewires market share is categorised into product, application and end user.
- The surgical guidewires segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The North America guidewires market is segmented by product into surgical guidewires and diagnostic guidewires. Among these, the surgical guidewires segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The growth of the segment is driven by the use of medical treatment methods where medical intervention is necessary to either cure or handle the disease. They allow for accurate positioning and steering of catheters, stents, balloons, and other therapeutic tools inside the human body.
- The coronary segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period.
Based on application, the North America guidewires market is segmented into coronary, neurovascular, urology, and others. Among these, the coronary segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period. Coronary guidewires were the largest segment in terms of revenue, accounting for 43.4% in 2023. Interventional cardiology makes a wide range of applications for these devices, including but not limited to angioplasty, stenting, thrombectomy, and fractional flow reserve. The National Library of Medicine reports that approximately 610,000 people die each year in the U.S. as a result of coronary artery disease.
- The hospitals segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The North America guidewires market is segmented by end user into hospitals, diagnostic centers and surgical centers, ambulatory care centers, and others. Among these, the hospitals segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The growth of the segment is driven by the coronary angiography, endoscopic retrograde cholangiopancreatography (ERCP), and several other vascular and urological diagnostic approaches. Furthermore, the use of guidewires extends to diagnostic imaging methods like angiography, which consists of the implantation of contrast materials into the blood vessels to see the blood circulation and find the irregularities.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the North America guidewires market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Boston Scientific Corporation
- Medtronic PLC
- Abbott Laboratories
- Terumo Corporation
- Stryker Corporation
- Cook Medical
- B. Braun Melsungen AG,
- Cardinal Health
- Teleflex Incorporated
- ASAHI INTECC
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
In October 2025, One Inc, a leading digital payments network for the insurance industry, announced the introduction of its flagship solution, ClaimsPay, to the Canadian property and casualty (P&C) market. The solution will be available as a Guidewire integration in Fall 2026 in the Guidewire Marketplace, marking an extension in the Guidewire and One Inc strategic partnership.
Market Segment
This study forecasts revenue at the North America, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the North America guidewires market based on the below-mentioned segments:
North America Guidewires Market, By Product
- Surgical Guidewires
- Diagnostic Guidewires
North America Guidewires Market, By Application
- Coronary
- Neurovascular
- Urology
- Others
North America Guidewires Market, By End User
- Hospitals
- Diagnostic Centers and Surgical Cenetrs
- Ambulatory Care Centers
- Others
Frequently Asked Questions (FAQ)
-
Q: What is the North America guidewires market size?A: The North America guidewires market size is expected to grow from USD 935.6 Million in 2024 to USD 1672.3 Million by 2035, growing at a CAGR of 5.42% during the forecast period 2025-2035.
-
Q: What are guidewires, and their primary use?A: A guidewire is a slender, adaptable wire usually composed of nitinol or stainless steel, and often the flexible tip configuration and specialized coatis are applied to it. The main purpose of the guidewire is to provide a safe temporary pathway.
-
Q: What are the key growth drivers of the market?A: Market growth is driven by the rising incidence of heart ailments, peripheral artery complications, and neurovascular disorders, which are forcing reliance on guidewire interventions.
-
Q: What factors restrain the North America guidewires market?A: The market is restrained by sophisticated guidewires are substantially costlier than traditional ones since they are composed of specific materials like hybrid polymers and nitinol.
-
Q: How is the market segmented by application?A: The market is segmented into coronary, neurovascular, urology, and others
-
Q: Who are the key players in the North America guidewires market?A: Key companies include Boston Scientific Corporation, Medtronic PLC, Abbott Laboratories, Terumo Corporation, Stryker Corporation, Cook Medical, B. Braun Melsungen AG, Cardinal Health, Teleflex Incorporated, and ASAHI INTECC.
Need help to buy this report?