Japan Genome Editing Market Size, Share, By Technology (CRISPR/Cas9, TALENs, Base Editing), By Delivery Method (Ex Vivo, In Vivo, In Situ), By Application (Genetic Engineering, Clinical Applications, Synthetic Biology), By Mode (Contract, In-house), By End Use (Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Agriculture, Industrial Biotechnology), Japan Genome Editing Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Genome Editing Market Insights Forecasts to 2035
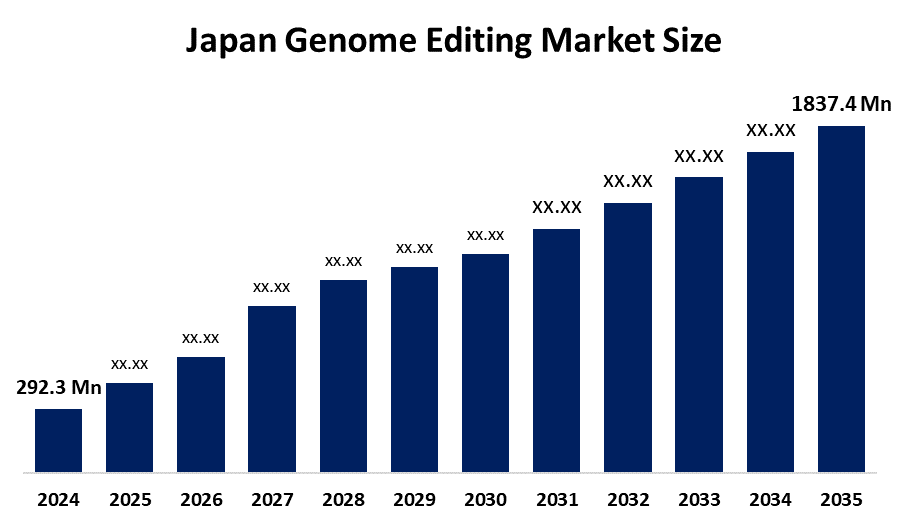
- Japan Genome Editing Market Size 2024: USD 292.3 Mn
- Japan Genome Editing Market Size 2035: USD 1837.4 Mn
- Japan Genome Editing Market CAGR 2024: 18.19%
- Japan Genome Editing Market Segments: By Technology, By Delivery Method, By Application, By Mode, and By End Use.
Get more details on this report -
The Japanese Genome Editing industry is comprised of accurate DNA modification tools, which include, among others, CRISPR/Cas9, TALENs, and still emerging base-editing tools, aimed at serving the pharmaceutical, biotechnology, agri, and research sectors for targeted gene correction, crop enhancement, and innovative bioscience-related research, respectively. These technologies are used in personalized medicine, for the treatment of rare genetic disorders, oncology therapies, for the creation of disease-resistant crops, for better food quality, for the improvement of aquaculture, and synthetic biology. This market is progressing owing to developments in CRISPR evolution (Cas3, base editing), AI-assisted bio-design, and enhanced delivery systems in lipid nanoparticles and adeno-associated viruses, among others.
Presently, initiatives by the government, such as the science and technology innovation promotion program (SIP) and the biotechnology strategy of Japan, are great sources of funding for these sectors, and in line with major developments in the field, countries have moved forward with a regulatory framework for the labelling of many gene-edited foods as non-GMO, ensuring quicker access to market. Future developments are bound by increasing demand for gene cell therapy, advancements in clinical studies, and access to investments in the field of biotechnology, among others.
Market Dynamics of the Japan Genome Editing Market:
The driving forces behind this market include the demand for personalized medicine, rare genetic diseases, and regenerative medicine. Government support in countries like Japan, with initiatives like the Science and Technology Innovation Promotion Program (SIP) and Japan’s biotechnology strategy, is a significant force. Advances in technology areas such as evolution in CRISPR, base editing, AI-assisted bio-designing, and delivery methods like lipid nanoparticles and Adeno-associated viral vectors are rapidly progressing gene and cell therapy and agricultural genomics.
The challenges faced by the market include ethics, strict regulation on the process of altering the human embryo, expenditure costs in relation to research and development, and restrictions in the acceptance level of genome-edited products. Technical issues, levels of scalability in trials, and the need for expertise are other drawbacks too.
The future prospects for the genome editing market in Japan are encouraging, and these include applications in the field of precision medicine, regenerative medicine, synthetic biology, industrial biotechnology, and genetically improved crops. This is attributed to an increasing number of clinical trials, venture capital investments, and favourable regulatory policies, among others.
Japan Genome Editing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 292.3 Million |
| Forecast Period: | 2024-2035 |
| Forecast Period CAGR 2024-2035 : | CAGR Of 18.19% |
| 2035 Value Projection: | USD 1837.4 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 210 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Application, By Technology |
| Companies covered:: | Merck KGaA Takara Bio Inc. Revvity, Inc. Danaher Corporation GenScript New England Biolabs Lonza Thermo Fisher Scientific, Inc. Charles River Laboratories Eurofins Scientific And Other Key Players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Market Segmentation
The Japan Genome Editing Market share is classified into technology, delivery method, application, mode, and end use.
By Technology:
The Japan Genome Editing Market Size is divided by technology into CRISPR/Cas9, TALENs, and base editing. Among these, the CRISPR/Cas9 segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. CRISPR/Cas9 leads the way mainly because of its high precision, wide range of applications in healthcare, agriculture, and research, strong academic, industry collaboration, and the regulatory support of non, GMO gene, edited products in Japan. Thus, it is naturally the choice for clinical and commercial uses.
By Delivery Method:
The market is divided by delivery method into ex vivo, in vivo, and in situ. Among these, the ex vivo segment dominated in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. Ex vivo methods give the most benefits because they offer the possibility to control the editing of cells outside the body, thus drastically reducing off, target effects. Consequently, patient safety is improved and, on top of that, ex vivo methods are thus the complex cell and gene therapies in hospitals and biotech facilities in Japan that use the most.
By Application:
The market is divided by application into genetic engineering, clinical applications, and synthetic biology. Among these, clinical applications dominated in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. The main factors driving this include demand for therapies for rare genetic diseases, cancer, and regenerative medicine as well as the number of clinical trials that utilize genome editing technology platforms to develop patient, specific treatments at a much faster pace.
By Mode:
The market is divided by mode into contract and in-house. Among these, the contract segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. By outsourcing, companies can tap into the expertise of a specialized partner, gain access to state, of, the, art infrastructure, accelerate R&D, etc. Hence, companies in Japan are able to reduce the time, to, market for their gene and cell therapy products.
By End Use:
The market is divided by end use into pharmaceutical & biotechnology companies, academic & research institutes, agriculture, and industrial biotechnology. Among these, pharmaceutical and biotechnology companies dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. The reason these companies have come out on top is that their increasing investments in gene and cell therapies, in precision medicine, clinical trials, and in commercial applications, have all fueled the need for advanced genome editing technologies in Japan.
Competitive Analysis:
The report offers the appropriate analysis of the key organisations/companies involved within the Japan Genome Editing Market Size, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
Top Key Companies in Japan Genome Editing Market:
- Merck KGaA
- Takara Bio Inc.
- Revvity, Inc.
- Danaher Corporation
- GenScript
- New England Biolabs
- Lonza
- Thermo Fisher Scientific, Inc.
- Charles River Laboratories
- Eurofins Scientific
Recent Developments in Japan Genome Editing Market:
In March 2025, GenScript Biotech Corp. announced that it has entered into a licensing deal with the Broad Institute, which will allow them to utilize the latter's prime editing technology. This move will not only amplify their commercial gene, editing capability but also pave the way for them to support more advanced therapeutic and research applications in Japan.
In January 2025, Takara Bio USA Holdings, Inc. has announced the acquisition of Curio Bioscience, which now allows the incorporation of Curio's Trekker and Seeker spatial, biology platforms into their single, cell omics portfolio to enhance the capabilities in precision medicine and gene research.
In March 2023, Thermo Fisher Scientific has joined forces with Arsenal Biosciences in a project to develop clinical, scale manufacturing processes for programmable autologous CAR, T cell therapies targeting platinum, resistant ovarian cancer. This collaboration will result in more efficient cell therapy production and better patient access in Japan.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan Genome Editing Market Size based on the below-mentioned segments:
Japan Genome Editing Market, By Technology
- CRISPR/Cas9
- TALENs
- Base Editing
Japan Genome Editing Market, By Delivery Method
- Ex Vivo
- In Vivo
- In Situ
Japan Genome Editing Market, By Application
- Genetic Engineering
- Clinical Applications
- Synthetic Biology
Japan Genome Editing Market, By Mode
- Contract
- In-house
Japan Genome Editing Market, By End Use
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
- Agriculture
- Industrial Biotechnology
Frequently Asked Questions (FAQ)
-
What is the Japan genome editing market?The Japan genome editing market involves precise DNA modification technologies such as CRISPR/Cas9, TALENs, and base editing, used across healthcare, agriculture, and research for applications including personalized medicine, rare genetic disorders, oncology therapies, regenerative medicine, crop improvement, and synthetic biology
-
What are the key applications of genome editing in Japan?Key applications include clinical applications for rare disease and cancer therapies, regenerative medicine, genetic engineering, synthetic biology, precision agriculture, improved food quality, aquaculture enhancement, and development of novel biopharmaceuticals
-
What are the main technologies used in Japan’s genome editing market?The main technologies include CRISPR/Cas9, TALENs, and base editing, with advanced CRISPR evolution like Cas3, AI-assisted bio-design, and delivery systems such as lipid nanoparticles and adeno-associated viral vectors
-
Who are the top companies operating in the Japan genome editing market?Top companies include Merck KGaA, Takara Bio Inc., Revvity Inc., Danaher Corporation, GenScript, New England Biolabs, Lonza, Thermo Fisher Scientific Inc., Charles River Laboratories, and Eurofins Scientific
-
What are the key drivers of the Japan genome editing market?Key drivers include rising demand for personalized medicine and gene therapies, government support through programs like the science and technology innovation promotion program (SIP) and the biotechnology strategy of Japan, technological advances, increasing private investments, and expansion of clinical trials
-
What are the major challenges in the Japan genome editing market?Challenges include ethical concerns, strict regulations on human embryo editing, high R&D costs, limited public acceptance of genome-edited products, technical complexities, scalability issues, and the need for specialized expertise
-
What future opportunities exist in the Japan genome editing market?Future opportunities include precision medicine, regenerative medicine, synthetic biology, industrial biotechnology, development of genetically improved crops, enhanced clinical trials, increased venture capital investments, and adoption of advanced technologies for efficient gene and cell therapies
Need help to buy this report?