Japan Early Toxicity Testing Market Size, Share By Technique (In Vivo, In Vitro and In Silico), By Toxicity Endpoint (Genotoxicity, Dermal Toxicity, Skin Toxicity, Ocular Toxicity, Phototoxicity, and Others), and By End-User (Pharmaceutical Industry, Cosmetic Industry, Chemical Industry, Food Industry, and Others), Japan Early Toxicity Testing Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Early Toxicity Testing Market Size Insights Forecasts to 2035
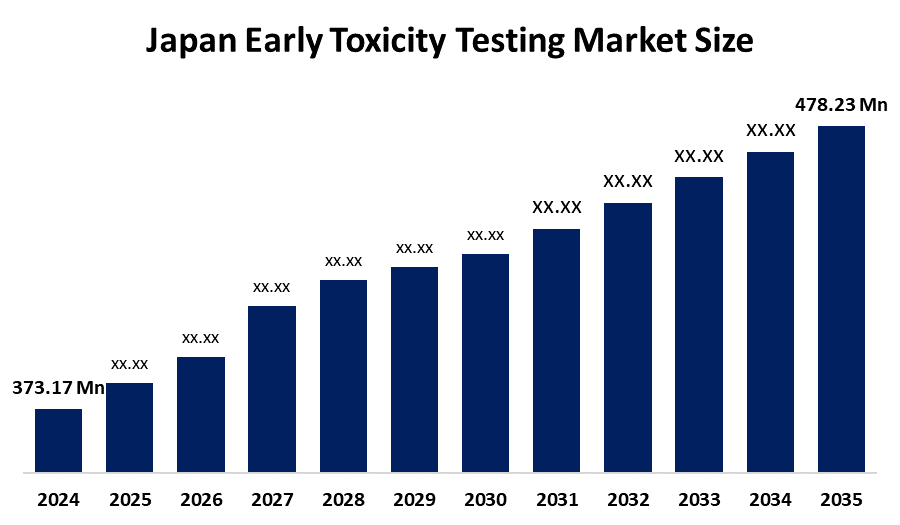
- Japan Early Toxicity Testing Market Size 2024: USD 373.17 Million
- Japan Early Toxicity Testing Market Size 2035: USD 478.23 Million
- Japan Early Toxicity Testing Market CAGR 2024: 2.28 %
- Japan Early Toxicity Testing Market Segments: Technique, Toxicity Endpoint, and End-User.
Get more details on this report -
The Japan Early Toxioty Testing Market Sue evaluates the potential harmful effects of chemicals, drugs, cosmetics, and food ingredients at early stages of development before clinical or commercial use. It includes in vivo, in vitro, and in silico methods in pharmaceutical, chemical, food, and cosmetic sectors to ensure safety, regulatory compliance, and reduce late-stage failures, supporting safer product development and public health protection, Furthermore, the Japan early toxicity testing market grows due to the expansion of the pharmaceutical industry and its strong R&D focus, requiring early safety evaluations to reduce drug failures. The rising incidence of cancer and chronic diseases Increases demand for safer therapies. Adoption of advanced technologies like 30 cell culture enhances prediction accuracy and drives broader use of early toxicity testing services.
Japan mandates the safety evaluation of chemicals and new substances under the Chemical Substances Control Law (CSCL) and related regulations. Manufacturers must submit toxicity data before approval, following Good Laboratory Practice (GLP standards and OECD test guidelines to harmonize methods globally. Regulatory bodies ensure testing protects human health and the environment, with periodic updates to toxicity assessment criteria and alternatives to animal testing.
The lapan early toxicity testing market trends include increased uptake of in vitro and in silico models, adoption of advanced 3D cell culture technologies, and growth in predictive toxicology tools. Emphasis on reducing animal testing and improving reliability supports modern testing techniques. Early toxicity testing expands beyond pharmaceuticals into cosmetics, food, and chemicals as safety awareness rises.
Market Dynamics of Japan Early Toxicity Testing Market:
The major drivers of Japan early toxicity testing market are regulatory compliance for emissions discharge, an increasing awareness of both workplace and environmental health, and the rising demand for high-purity filtration Toxicity Endpoints in pharmaceutical and electronics manufacturing. The need for companies to grow through industrial expansion and to meet their sustainability goals is motivating companies to update and improve their filtration infrastructure. The continued focus on reducing operational costs through more efficient filtration solutions will also help support the growth of the market as well as technological innovations.
Japan early toxicity testing market testing infrastructure and technologies hinder market growth in Japan, especially for smaller organisations with limited resources. Complex and expensive compliance procedures for regulatory safety evaluation also slow adoption. Moreover, lengthy and resource-intensive tests restrict rapid throughput, limiting broader implementation despite demand.
Japan early toxicity testing market presents opportunities include expanding services in emerging technologies like 30 cell cultures, organ-on-chip systems, and predictive in silico models that enhance accuracy and reduce costs. Growing pharmaceutical R&D in Japan, rising safety expectations, shifts toward non-animal testing, and collaborations among CROs can further stimulate market expansion and innovation, especially in high-precision pre-clinical assessments.
Market Segmentation
Japan early toxicity testing market share is classified into technique, toxicity endpoint, and end-user.
By Technique:
Japan early toxicity testing market is divided by technique into in vivo, in vitro and in silico. Among these, the in vitro segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. The in vitro segment dominates because it is faster, cost-effective, and ethically preferred. Advanced technologies like 3D cell cultures and organ-on-chip models, along with regulations reducing animal testing, drive widespread adoption in pharmaceuticals, cosmetics, and chemical safety evaluations.
By Toxicity Endpoint:
Japan early toxicity testing market is divided by toxicity endpoint into genotoxicity, dermal toxicity, skin toxicity, ocular toxicity, phototoxicity, and others. Among these, the genotoxicity segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. The genotoxicity segment dominates because detecting DNA damage is crucial for safety. Regulatory requirements prioritize it, and pharmaceutical, chemical, and cosmetic companies widely adopt these tests to ensure compliance and prevent late-stage failures.
By End-User
Japan early toxicity testing market is divided by end-user into pharmaceutical industry, cosmetic industry, chemical industry, food industry, and others. Among these, the pharmaceutical industry segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. The pharmaceutical industry segment is dominant because drugs require extensive early toxicity testing to ensure safety, meet strict regulations, reduce clinical failures, and adopt advanced in vitro and in silico methods, driving high demand in Japan’s preclinical testing market.
Competitive Analysis:
The report offers the appropriate analysis of the key organisations/companies involved within Japan early toxicity testing market, along with a comparative evaluation primarily based on their Toxicity Endpoint offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes Toxicity Endpoint development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
Top Key Companies in Japan Early Toxicity Testing Market:
- Merck KGaA
- Laboratory Corporation of America Holdings
- WuXi AppTec
- Medpace
- Thermo Fisher Scientific
- Eurofins Scientific
- PerkinElmer, Inc.
- Bio-Rad Laboratories
- Agilent Technologies
- Bruker Corporation
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented Japan early toxicity testing market based on the following segments:
Japan Early Toxicity Testing Market, By Technique
- In Vivo
- In Vitro
- In Silico
Japan Early Toxicity Testing Market, By Toxicity Endpoint
- Genotoxicity
- Dermal Toxicity
- Skin Toxicity
- Ocular Toxicity
- Phototoxicity
- Others
Japan Early Toxicity Testing Market By End-User
- Pharmaceutical Industry
- Cosmetic Industry
- Chemical Industry
- Food Industry
- Others
Frequently Asked Questions (FAQ)
-
Q1: What is Japan early toxicity testing market? Japan early toxicity testing market is expected to grow from USD 373.17 million in 2024 to USD 478.23 million by 2035, growing at a CAGR of 2.28 % during the forecast period 2025-2035.
-
Q 2: What are the key growth drivers of Japan early toxicity testing market? Japan early toxicity testing market's Key growth drivers are rising pharmaceutical R&D, demand for safer drugs, chronic disease prevalence, advanced technologies, and strict regulatory requirements.
-
Q 3: What factors restrain Japan early toxicity testing market? Constraints include market restraints include high testing costs, complex regulatory compliance, limited skilled workforce, lengthy procedures, and infrastructure challenges in smaller organisations.
-
Q 4: How is Japan early toxicity testing market segmented by end-user? Japan early toxicity testing market includes Pharmaceutical Industry, Cosmetic Industry, Chemical Industry, Food Industry, and Others
-
Q 5: Who are the key players in Japan early toxicity testing market? Key players in the Japan early toxicity testing market include Merck KGaA, LabCorp, Wuxi Apptec, Medpace, Thermo Fisher Scientific, Eurofins Scientific, PerkinElmer, Bio-Rad, Agilent Technologies, and Bruker Corporation
Need help to buy this report?