Japan Clinical Trials Support Services Market Size, Share, By Phase Type (Phase I, Phase II, Phase III, and Phase IV), By Service (Clinical Trial Site Management, Patient Recruitment Management, Data Management, Administrative Staff, IRB, and Others), Japan Clinical Trials Support Services Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Clinical Trials Support Services Market Insights Forecasts to 2035
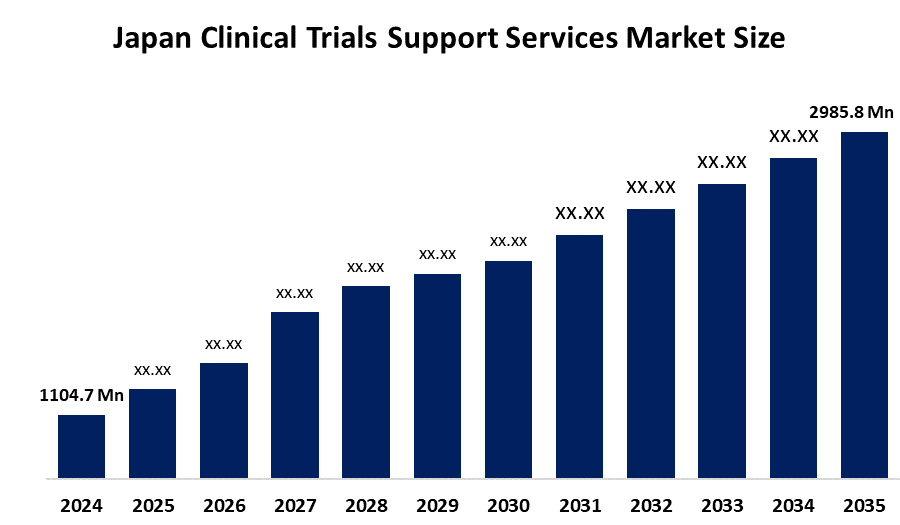
- Japan Clinical Trials Support Services Market Size 2024: USD 1104.7 Mn
- Japan Clinical Trials Support Services Market Size 2035: USD 2985.8 Mn
- Japan Clinical Trials Support Services Market CAGR 2024: 9.46%
- Japan Clinical Trials Support Services Market Segments: Phase Type and Service
Get more details on this report -
The Japan Clinical Trials Support Services Market Size is made up of the services that are specially designed to help the pharmaceutical, biotechnology, and research organizations in planning, managing, and conducting clinical trials through all the phases while guaranteeing regulatory compliance, patient safety, data integrity, and fast drug development in Japan. The growth of the market is backed by the increase in the number of clinical trials performed in Japan, more and more global sponsors taking advantage of the excellent healthcare system in the region, and the trend of outsourcing specialized support services to be quick and to comply with the regulations.
The clinical trials in Japan are backed by government support, including the AMED funding programs, which help to improve trial infrastructure, site networks, and staff training. The regulatory incentives are given through the policies of Sakigake and Orphan Drug designations, whereas the national biotechnology investments of about ¥500 billion are boosting the clinical research capacities and outsourcing support services.
As technology advances, Japanese clinical trial support providers are now using electronic data capturing (EDC), telehealth, and artificial intelligence (AI) to increase efficiency, expedite recruitment, and create higher-quality data. This new 'hybrid/decentralised' clinical trial process allows for enhanced access for patients. By utilizing advanced analytical and predictive models, as well as real-world evidence, these providers can also ensure compliance with regulatory requirements and successfully perform adaptive clinical trial designs.
Market Dynamics of the Japan Clinical Trials Support Services Market:
The Japan Clinical Trials Support Services Market Size is driven by the rise in pharmaceutical and biotechnology R&D activities, the increase in the number of chronic and rare disease cases, and the demand for quicker drug development. The regulatory standards and intricate trial designs make it necessary for companies to outsource to specialized service providers. Besides the government's actions in favor of clinical research, the introduction of digital and decentralized trial models, and the increasing use of AI, data analytics, and e-clinical platforms are the factors that will continue to push the market forward.
The Japan Clinical Trials Support Services Market Size is restrained by the high costs of operation and compliance, long regulatory approval periods, a small number of patients that can participate in the trials, difficulties in communication and culture, and a lack of experienced professionals in clinical research, all of which result in slowing down the execution of trials and increasing the total cost of development.
The future of Japan's clinical trial support services market is bright and promising, with versatile opportunities emerging from the rise of decentralized and hybrid trials, AI-assisted patient enrollment, real-world evidence creation, and digital health combo. Furthermore, the incorporation of new technologies such as e-clinical platforms, wearables, and predictive analytics will optimize the overall trial process, make patients more involved, and also meet regulatory requirements.
Market Segmentation
The Japan Clinical Trials Support Services Market share is classified into phase type and service.
By Phase Type:
The Japan Clinical Trials Support Services Market Size is divided by phase type into phase I, phase II, phase III, and phase IV. Among these, the phase III segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. Large patient populations, longer study durations, increased complexity, and strict regulatory criteria all contribute to the Phase III segment's dominance and higher spending on clinical trial support services when compared to previous trial stages.
By Service:
The Japan clinical trials support services market is divided by service into clinical trial site management, patient recruitment management, data management, administrative staff, IRB, and others. Among these, the data management segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. The data management segment dominates because of expanding clinical trial complexity, huge amounts of patient and regulatory data, tight compliance requirements, and increasing usage of digital platforms for real-time data gathering, monitoring, and analysis in Japan.
Competitive Analysis:
The report offers the appropriate analysis of the key organisations/companies involved within the Japan clinical trials support services market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
Top Key Companies in Japan Clinical Trials Support Services Market:
- CMIC Group
- EPS Corporation
- PRA Health Sciences Japan
- I’rom Group
- Cliantha Research Japan
- Shonan Health Innovation Park
- Translational Research Informatics Center (TRI)
- Medical Network Systems (MNS)
- Others
Recent Developments in Japan Clinical Trials Support Services Market:
In July 2025, A strategic partnership between IQVIA Services Japan and the Cancer Institute Hospital of JFCR was established to improve the execution of oncology clinical trials and to broaden the network of trials in Japan.
In July 2025, Verified Clinical Trials (VCT), together with Medical Revolutions Japan (MRJ) and JACIC, started to operate in Japan officially to avoid duplicate and professional subjects in clinical trials.
In August 2024, Fujitsu introduced a new AI-enabled Patient-centric Clinical Trials service and teamed up with Paradigm Health to speed up the process of clinical trial digitalization in Japan, which included both the automation of documentation and planning workflows.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan clinical trials support services market based on the below-mentioned segments:
Japan Clinical Trials Support Services Market, By Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
Japan Clinical Trials Support Services Market, By Service
- Clinical Trial Site Management
- Patient Recruitment Management
- Data Management
- Administrative staff
- IRB
- Others
Frequently Asked Questions (FAQ)
-
Q: What is the Japan clinical trials support services market size?A: Japan Clinical Trials Support Services Market is expected to grow from USD 1104.7 million in 2024 to USD 2985.8 million by 2035, growing at a CAGR of 9.46% during the forecast period 2025-2035.
-
Q: What are the key growth drivers of the market?A: Market growth is driven by the rise in pharmaceutical and biotechnology R&D activities, the increase in the number of chronic and rare disease cases, and the demand for quicker drug development. The regulatory standards and intricate trial designs make it necessary for companies to outsource to specialized service providers. Besides the government's actions in favor of clinical research, the introduction of digital and decentralized trial models, and the increasing use of AI, data analytics, and e-clinical platforms are the factors that will continue to push the market forward.
-
Q: What factors restrain the Japan clinical trials support services market?A: Constraints include the high costs of operation and compliance, long regulatory approval periods, a small number of patients that can participate in the trials, difficulties in communication and culture, and a lack of experienced professionals in clinical research, all of which result in slowing down the execution of trials and increasing the total cost of development.
-
Q: How is the market segmented by phase type?A: The market is segmented into phase I, phase II, phase III, and phase IV.
-
Q: Who are the key players in the Japan clinical trials support services market?A: Key companies include CMIC Group, EPS Corporation, PRA Health Sciences Japan, I’rom Group, Cliantha Research Japan, Shonan Health Innovation Park, Translational Research Informatics Center (TRI), Medical Network Systems (MNS), and Others.
-
Q: Who are the target audiences for this market report?A: The report targets market players, investors, end-users, government authorities, consulting and research firms, venture capitalists, and value-added resellers (VARs).
Need help to buy this report?