Japan Clinical Trials Management System Market Size, Share, and COVID-19 Impact Analysis, By Component (Software, Services), By Deployment Mode (Web-based CTMS, On-premises, Cloud-based CTMS), By End User (Pharmaceutical and Biotechnology Firms, Contract Research Organizations, and Others), and Japan Clinical Trials Management System Market Size Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Clinical Trials Management System Market Size Insights Forecasts to 2035
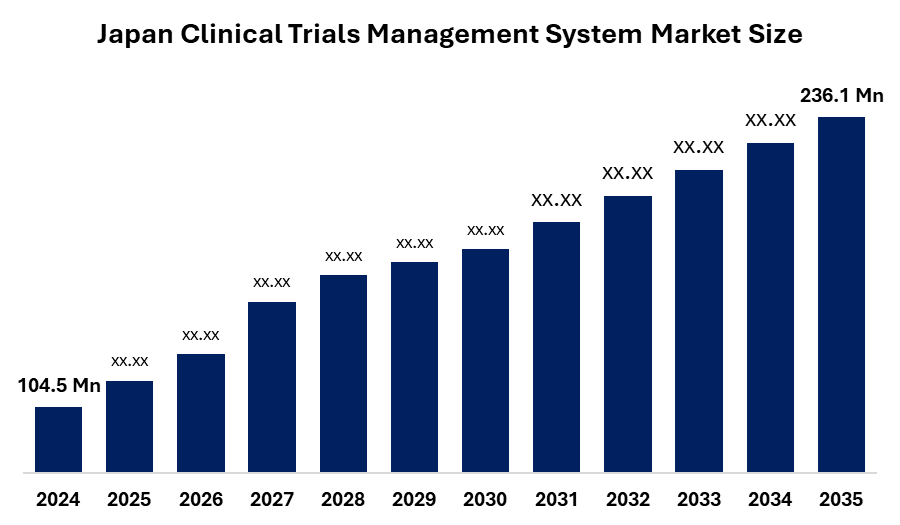
- The Japan Clinical Trials Management System Market Size was estimated at USD 104.5 Million in 2024
- The Market Size is Expected to Grow at a CAGR of Around 7.69% from 2025 to 2035
- The Japan Clinical Trials Management System Market Size is Expected to Reach USD 236.1 Million by 2035
Get more details on this report -
According to a Research Report Published by Spherical Insights & Consulting, The Japan Clinical Trials Management System Market Size is Anticipated to Reach USD 236.1 Million by 2035, Growing at a CAGR of 7.69% from 2025 to 2035. The market is driven by the rising number of clinical trials, increasing adoption of digital solutions in healthcare research, and the growing need for regulatory compliance and data transparency. The shift toward cloud-based platforms and the integration of advanced analytics are further accelerating market growth.
Market Overview
The Japan Clinical Trials Management System (CTMS) Market Size refers to software solutions aimed at controlling, monitoring, and simplifying clinical trial processes, such as preparation, patient recruitment, data handling, compliance, and reporting. CTMS systems lift the overall output of the operation, lower mistakes, and make sure that laws are followed. The main trends are the use of cloud-based CTMS, the connection with electronic data capture systems, and the growing application of real time analytics. Among the market features are considerable regulatory inspection, rapid acceptance of technology, and the need for automation. The most significant makings are growing R&D expenditures, more intricate clinical trials, and faster drug development timelines. The market for centralized and efficient trial management tools is growing due to the increasing complexity of clinical trials, which include multi-site studies, large data volumes, and strict timelines. Moreover, there is a shift in focus towards patient centric trial management, where tools that enable remote visits and better communication with trial participants are being used. The market is gradually moving toward more integrated, data-driven, and compliant CTMS platforms to support the expanding clinical research ecosystem in Japan.
The government of Japan is backing clinical research and the use of digital healthcare by setting up initiatives that would lead to the innovation of pharmaceuticals, regulatory harmonization, and the transformation of the healthcare system through digital means. Programs that promote the use of clinical trial technologies by medical research, the use of real-world data, and collaboration between the academic and industry sectors are gaining strength.
Report Coverage
This research report categorizes the market for the Japan Clinical Trials Management System Market Size (CTMS) based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan clinical trials management system (CTMS) market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan clinical trials management system (CTMS) market.
Japan Clinical Trials Management System Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | 104.5 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR of 7.69% |
| 2035 Value Projection: | 236.1 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 240 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Component, By Deployment Mode |
| Companies covered:: | Oracle Corporation, Medidata Solutions (Dassault Systemes), Veeva Systems, Parexel International Corporation, ICON plc, BioClinica, Fujitsu Limited, and other key players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The Japan Clinical Trials Management System Market Size is driven by the upsurge in clinical trials, escalating pharmaceutical and biotech R&D costs, and the necessity for effective trial monitoring, which are the main factors driving the market. The application of advanced CTMS solutions is facilitated by regulatory standards for data reliability, patient security, and conformity. Furthermore, the transition to decentralized and multicentre clinical trials, together with the need for immediate access to data and automation of workflows, is a major factor supporting the market's expansion.
Restraining Factors
The Japan Clinical Trials Management System Market Size faces profound implementation costs, worries about data security, and the difficulty of merging with the older healthcare IT systems, which are the main reasons that hold back the developers. The lack of sufficient technical know how at the smaller research organizations may also be a factor in adoption.
Market Segmentation
The Japan clinical trials management system market share is categorized by component, deployment mode, and end user.
- The software segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan Clinical Trials Management System Market Size is segmented by component into software and services. Among these, the software segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The segmental growth is driven by its capability to unify trial procedures, transition tasks to machines, and maintain adherence to laws. The CTMS solution assists in the planning, financial controlling, site handling, and data dissemination of clinical trials, thus being indispensable for the quick and smooth running of trials.
- The cloud-based CTMS segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan Clinical Trials Management System Market Size is segmented by deployment mode into web-based CTMS, on-premises, and cloud-based CTMS. Among these, the cloud-based CTMS segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The segmental growth is driven by scalability, cost efficiency, remote accessibility, and the ability to easily integrate with other clinical systems were the main advantages of cloud solutions. Moreover, these solutions facilitated instant collaboration among several trial locations.
- The pharmaceutical and biotechnology firms segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan Clinical Trials Management System Market Size is segmented by end user into pharmaceutical and biotechnology firms, contract research organizations, and others. Among these, the pharmaceutical and biotechnology segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The segmental growth is driven by the companies' vast clinical pipelines, large-scale trial operations, and main objective to fast-track drug development with complete adherence to strict regulations, which have all contributed to their success.
Competitive Analysis
The report offers the appropriate analysis of the key organizations/companies involved within the Japan clinical trials management system market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Oracle Corporation
- Medidata Solutions (Dassault Systemes)
- Veeva Systems
- Parexel International Corporation
- ICON plc
- BioClinica
- Fujitsu Limited
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan Clinical Trials Management System Market based on the below-mentioned segments:
Japan Clinical Trials Management System Market, By Component
- Software
- Services
Japan Clinical Trials Management System Market, By Deployment Mode
- Web-based CTMS
- On-premises
- Cloud-based CTMS
Japan Clinical Trials Management System Market, By End User
- Pharmaceutical and Biotechnology Firms
- Contract Research Organizations
- Others
Frequently Asked Questions (FAQ)
-
What is the Japan clinical trials management system market size?The market is expected to grow from USD 104.5 million in 2024 to USD 236.1 million by 2035, at a CAGR of 7.69% during 2025 to 2035.
-
What are the key growth drivers of the market?Growth is driven by increasing clinical trials, rising R&D investments, regulatory compliance requirements, and adoption of cloud-based CTMS solutions.
-
What factors restrain the Japan clinical trials management system market?High implementation costs, data security concerns, and system integration challenges restrain market growth.
-
How is the market segmented by component?The market is segmented into software and services.
-
Who are the key players in the Japan clinical trials management system market?Key players include Oracle Corporation, Medidata Solutions, Veeva Systems, Parexel International, ICON plc, and Fujitsu Limited.
Need help to buy this report?