Japan Cervical Cancer Diagnostic Market Size, Share, By Test Type (Pap Smear Test, HPV DNA Test, HPV mRNA Test, Co-testing, Visual Inspection with Acetic Acid [VIA], Visual Inspection with Lugol’s Iodine [VILI], Colposcopy, Cervical Biopsy, Endocervical Curettage [ECC], and Others), By End User (Hospitals, Diagnostic Centers, Specialty Clinics, Cancer & Radiation Therapy Centers, and Others), Japan Cervical Cancer Diagnostic Market Insights, Industry Trends, Forecasts to 2035
Industry: HealthcareJapan Cervical Cancer Diagnostic Market Size Insights Forecasts To 2035
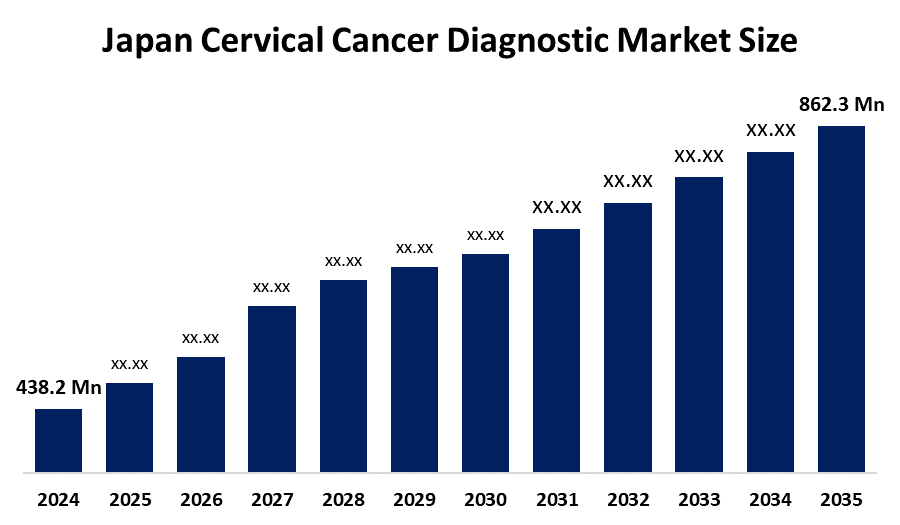
- Japan Cervical Cancer Diagnostic Market Size 2024: USD 438.2 Mn
- Japan Cervical Cancer Diagnostic Market Size 2035: USD 862.3 Mn
- Japan Cervical Cancer Diagnostic Market Size CAGR 2024: 6.35%
- Japan Cervical Cancer Diagnostic Market Size Segments: Test Type and End User
Get more details on this report -
The Cervical Cancer Diagnostic Market Size In Japan Can Be Described As The Cervical Cancer Screening Tests/Diagnostic Examinations Used To Identify Cell Abnormalities And Cervical Cancer In The Early Stages. This can be divided into cytological screening tests, HPV molecular screening tests, colposcopy examinations, and biopsy examinations. For the Japanese market, the demand for the cervical cancer screening/diagnostic market can be explained as being fueled by the implementation of screening programs in the country and the rising adoption of screening examinations in the country.
The Market Size In Japan Is Greatly Influenced By The Government’s Initiatives. The ministry of health, labour, and welfare resumed active HPV vaccination recommendations, including HPV vaccination in the National Immunization Program in April 2023, alongside HPV “catch-up vaccination” programs for women aged between 1997 and 2007. The national guidelines include “biennial cervical cytology screening for women aged 20 years and above.” Advances in technology have been witnessed through a shift to liquid-based cytology techniques, HPV DNA testing, HPV mRNA testing, digital colposcopy, and other techniques. New opportunities include the development of HPV-based primary screening, AI image analysis, HPV-based organized screening, increased capacities for early detection that are related to Japan’s national cancer control plan, and other techniques.
Japan Cervical Cancer Diagnostic Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 438.2 Million |
| Forecast Period: | 2020-2023 |
| Forecast Period CAGR 2020-2023 : | 6.35% |
| 2023 Value Projection: | USD 862.3 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 250 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Test Type, By End User |
| Companies covered:: | Hologic Japan Co., Ltd., QIAGEN Japan Co., Ltd., Abbott Japan LLC, Becton, Dickinson and Company Japan, Roche Diagnostics K.K., Sysmex Corporation, Fujirebio Inc., Olympus Corporation, Canon Medical Systems Corporation, and Others, Key Players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Market Dynamics of the Japan Cervical Cancer Diagnostic Market:
The japan cervical cancer diagnostic market size is also driven by initiatives taken by the government to enhance low levels of screening,the renewed promotion of active promotion of HPV vaccination by the Ministry of Health, Labour, and Welfare, as well as cervical cancer screening guidelines mandated across the nation targeting women aged 20 years and above. The market is also expected to be fueled by the usage of HPV DNA tests, liquid cytology, as well as colposcopy procedures.
The market size is restrained by low historically recorded screening participation ratesgeographical variations in accessibility of organized screening programs, workforce skills deficiency in cytology, and higher costs of complex molecular diagnostic techniques. Also, differences in screening rates among age groups and reliance on an opportunistic approach can be hindrances.
The future outlook of the japan cervical cancer diagnostic market size appears promising,, driven by continued growth in HPV-based primary screening, inclusion of molecular diagnostics in national screening programs, digital-colposcopy technology, and early cancer detection efforts in line with the national cancer solution strategy in Japan. Improvement in infrastructure related to organized screening will continue to create growth opportunities in Japan’s cervical cancer diagnostic market.
Market Segmentation
The Japan cervical cancer diagnostic market share is classified into test type and end user.
By Test Type:
The japan cervical cancer diagnostic market size is dividedBy Test Type Into Pap Smear Test, HPV DNA Test, HPV mRNA Test, Co-testing, VIA, VILI, colposcopy, cervical biopsy, ECC, and others. Among these, the HPV DNA Test segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. The growth will be orchestrated by better sensitivity for high-risk HPV identification, pre-cancerous lesion detection at an early stage, harmonization with country-level screening guidelines, automation possibilities, scalability for laboratory implementation, reimbursement acceptance, fewer false negatives, and rising prominence compared to cyto logical primary screening methods.
By End User:
The Japan cervical cancer diagnostic market size is divided by end user into hospitals, diagnostic centers, specialty clinics, cancer & radiation therapy centers, and others. Among these, the Hospitals segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. Growth is fueled by high volumes of patients, access to comprehensive diagnostic resources, presence of a gynaecologist as well as an oncologist, participation by the population in governmental screening services, availability of biopsy as well as colposcopy resources, an organized treatment chain, inpatient diagnostic resources, and the concentration of patients from sources such as primary health care.
Competitive Analysis:
The report offers the appropriate analysis of the key organisations/companies involved within the japan cervical cancer diagnostic market size, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
Top Key Companies in Japan Cervical Cancer Diagnostic Market:
- Hologic Japan Co., Ltd.
- QIAGEN Japan Co., Ltd.
- Abbott Japan LLC
- Becton, Dickinson and Company Japan
- Roche Diagnostics K.K.
- Sysmex Corporation
- Fujirebio Inc.
- Olympus Corporation
- Canon Medical Systems Corporation
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan cervical cancer diagnostic market based on the below-mentioned segments:
Japan Cervical Cancer Diagnostic Market, By Test Type
- Pap Smear Test
- HPV DNA Test
- HPV mRNA Test
- Co-testing
- Visual Inspection with Acetic Acid (VIA)
- Visual Inspection with Lugol’s Iodine (VILI)
- Colposcopy
- Cervical Biopsy
- Endocervical Curettage (ECC)
- Others
Japan Cervical Cancer Diagnostic Market, By End User
- Hospitals
- Diagnostic Centers
- Specialty Clinics
- Cancer & Radiation Therapy Centers
- Others
Frequently Asked Questions (FAQ)
-
What is the Japan cervical cancer diagnostic market size?The Japan cervical cancer diagnostic market is expected to grow from USD 438.2 million in 2024 to USD 862.3 million by 2035, registering a CAGR of 6.35% during the forecast period 2025-2035.
-
What are the key growth drivers of the Japan cervical cancer diagnostic market?Market growth is driven by government-led HPV vaccination resumption, national cervical screening guidelines, increasing adoption of HPV DNA and liquid-based cytology tests, rising awareness of early detection, and technological advancements such as molecular diagnostics and digital colposcopy in Japan
-
What factors restrain the Japan cervical cancer diagnostic market?Market growth is restrained by historically low screening participation rates, uneven access to organized screening programs, shortages of skilled cytology professionals, higher costs of advanced molecular diagnostics, and continued reliance on opportunistic screening practices across regions.
-
How is the Japan cervical cancer diagnostic market segmented?The market is segmented by test type and end user.
-
Which test type segment dominates the Japan cervical cancer diagnostic market?The HPV DNA Test segment dominated the market share in 2024 due to higher sensitivity, early detection of high-risk HPV strains, compatibility with automation, reimbursement acceptance, and increasing alignment with national screening recommendations.
-
Which end user segment holds the largest share in the market?Hospitals dominated the market share in 2024 owing to high patient volumes, availability of gynaecology and oncology specialists, access to colposcopy and biopsy infrastructure, and participation in government-supported cervical screening programs.
-
Who are the key players in the Japan cervical cancer diagnostic market?Key companies operating in the market include Hologic Japan Co., Ltd., QIAGEN Japan Co., Ltd., Abbott Japan LLC, Becton, Dickinson and Company Japan, Roche Diagnostics K.K., Sysmex Corporation, Fujirebio Inc., Olympus Corporation, Canon Medical Systems Corporation, and others.
-
Who are the target audiences for the Japan cervical cancer diagnostic market report?The report targets market players, investors, end users, government authorities, consulting and research firms, venture capitalists, and value-added resellers (VARs).
Need help to buy this report?