France Hernia Repair Devices Market Size, Share, By Product (Hernia Mesh and Hernia Fixation Devices), By Procedure Type (Open Surgery and Laparoscopic Surgery), By Surgery Type (Inguinal Hernia, Umbilical Hernia, Incisional Hernia, Femoral Hernia, and Others), France Hernia Repair Devices Market Insights, Industry Trend, Forecasts to 2035.
Industry: HealthcareFrance Hernia Repair Devices Market Insights Forecasts to 2035
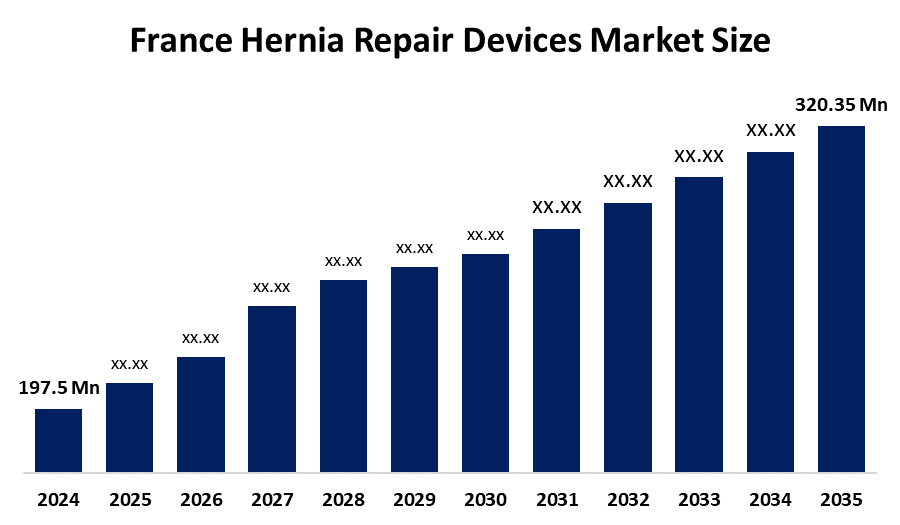
- France Hernia Repair Devices Market Size 2024: USD 197.5 Mn
- France Hernia Repair Devices Market Size 2035: USD 320.35 Mn
- France Hernia Repair Devices Market CAGR 2024: 4.5%
- France Hernia Repair Devices Market Segments: Product, Procedure Type, and Surgery Type
Get more details on this report -
The France Hernia Repair Devices Market Size forms an integral part of the country’s medical device industry and includes a wide range of devices used to provide surgical solutions for the treatment of various types of hernias. These solutions encompass hernia meshes, fixation systems, and laparoscopic and open repair systems, which are utilized across hospitals and surgical centers to deliver effective patient care. Market growth is driven by the high volume of hernia procedures performed in France, a rapidly aging population, and the increasing adoption of minimally invasive surgical techniques. These trends are further supported by France’s advanced healthcare infrastructure and a well-established reimbursement system.
The French government has introduced multiple initiatives to promote clinical research and accelerate the adoption of advanced hernia repair technologies. Under the France 2030 investment plan, approximately €7 billion has been allocated to healthcare research and development, with around USD 400 million specifically dedicated to the development of innovative medical devices. In addition, funding through programs such as the STIC fund supports clinical trials and enhances hospital-based research infrastructure by strengthening clinical trial networks. Combined with supportive European Union regulatory pathways and continued public investment in healthcare, these initiatives significantly improve clinical research capacity and speed up the adoption of next-generation hernia repair devices.
Looking ahead, the France hernia repair devices market is expected to benefit from emerging growth opportunities driven by technological advancements. These include the development of lightweight and bioabsorbable mesh products, innovations in fixation devices that allow improved tension control, and the growing adoption of robot-assisted and minimally invasive surgical procedures. Together, these advancements are anticipated to support sustained market growth in the coming years.
France Hernia Repair Devices Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 197.5 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR of 4.5% |
| 2035 Value Projection: | USD 320.35 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 158 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Product, By Procedure Type, By Surgery Type |
| Companies covered:: | Medtronic plc, Johnson & Johnson (Ethicon), Becton Dickinson (BD), W. L. Gore & Associates, Inc., B. Braun SE, Cook Medical (Cook Group), Herniamesh S.r.l., Atrium Medical (Getinge Group), Cousin Biotech (France), LifeCell International, Smith+Nephew, Stryker, Conmed, Integra LifeSciences,and Others key players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Market Dynamics of the France Hernia Repair Devices Market
The France Hernia Repair Devices Market Size is driven by several key factors, including the high number of patients diagnosed with hernias, a growing elderly population at increased risk of hernia development, and improved access to medical care supported by a robust healthcare system. Additionally, the rising adoption of minimally invasive and endoscopic surgical techniques has significantly contributed to market growth. Continuous advancements in hernia mesh materials and fixation devices further support the expansion of this market.
Despite these positive drivers, the market faces certain restraints. The high cost of advanced hernia repair devices and associated surgical procedures can limit adoption among healthcare providers. Potential postoperative complications, such as infections, mesh rejection, and hernia recurrence, may also influence device selection. Furthermore, the high cost of innovative devices can delay market entry and slow overall market growth. Limited awareness and insufficient training related to newer technologies among some healthcare professionals may further restrict widespread adoption.
Market Segment:
The France Hernia Repair Devices Market is segmented by Product, Procedure Type, and Surgery Type.
By Product:
The market is segmented into hernia mesh and hernia fixation devices. Among these, the hernia mesh segment dominated the market share in 2024 and is expected to grow at a notable CAGR during the forecast period. Growth in this segment is driven by the high volume of hernia surgeries, advancements in mesh materials such as lightweight, biologic, and bioresorbable options, increasing use of minimally invasive procedures, surgeon preference for durable solutions, and favorable government reimbursement policies in France.
By Procedure Type:
Based on procedure type, the market is segmented into open surgery and laparoscopic surgery. The open surgery segment held the largest market share in 2024 and is projected to grow at a strong CAGR during the forecast period. This dominance is attributed to its widespread use in treating complex and recurrent hernias, strong surgeon familiarity, lower equipment requirements, and well-established clinical protocols, making it a preferred approach in many French hospitals.
By Surgery Type:
The market is segmented into inguinal hernia, umbilical hernia, incisional hernia, femoral hernia, and others. Among these, the inguinal hernia segment dominated the market share in 2024 and is anticipated to grow at a significant CAGR during the forecast period. This segment’s dominance is driven by the high prevalence of inguinal hernias the most common type among adults along with a large number of procedures performed annually, standardized treatment protocols, and the availability of effective repair devices such as advanced meshes and fixation systems that ensure improved patient outcomes and reduced recurrence rates.
Competitive Analysis
The report provides a comprehensive analysis of key organizations operating in the France hernia repair devices market, along with a comparative evaluation based on product offerings, business overviews, geographic presence, corporate strategies, segment-wise market share, and SWOT analysis. It also includes an in-depth assessment of recent company developments, such as product launches, technological innovations, joint ventures, partnerships, mergers and acquisitions, strategic alliances, and other strategic initiatives. This enables a clear evaluation of the overall competitive landscape within the market.
Top Key Companies in the France Hernia Repair Devices Market
- Medtronic plc
- Johnson & Johnson (Ethicon)
- Becton Dickinson (BD)
- W. L. Gore & Associates, Inc.
- B. Braun SE
- Cook Medical (Cook Group)
- Herniamesh S.r.l.
- Atrium Medical (Getinge Group)
- Cousin Biotech (France)
- LifeCell International
- Smith+Nephew
- Stryker
- Conmed
- Integra LifeSciences
- Others
Recent Developments in the France Hernia Repair Devices Market
In April 2025, BD launched the Phasix™ ST Umbilical Hernia Patch, the first fully bioabsorbable mesh designed specifically for umbilical hernia repair. The product received FDA 510(k) clearance and expands advanced hernia repair options through absorbable biomaterial technology.
Key Target Audience
- Market Players
- Investors
- End Users
- Government Authorities
- Consulting and Research Firms
- Venture Capitalists
- Value-Added Resellers (VARs)
Market Segmentation
This study forecasts revenue for the France hernia repair devices market at the national, regional, and country levels from 2020 to 2035.Spherical Insights has segmented the market based on the following criteria:
France Hernia Repair Devices Market, By Product
-
·Hernia Mesh
-
Hernia Fixation Devices
France Hernia Repair Devices Market, By Procedure Type
-
Open Surgery
-
Laparoscopic Surgery
France Hernia Repair Devices Market, By Surgery Type
-
Inguinal Hernia
-
Umbilical Hernia
-
Incisional Hernia
-
Femoral Hernia
-
Others
Frequently Asked Questions (FAQ)
-
1. What is the France hernia repair devices market size in 2024?The France hernia repair devices market size was estimated at USD 197.5 million in 2024.
-
2. What is the projected market size of the France hernia repair devices market by 2035?The France hernia repair devices market size is expected to reach USD 320.35 million by 2035.
-
3. What is the CAGR of the France hernia repair devices market?The France hernia repair devices market size is expected to grow at a CAGR of around 4.5% from 2024 to 2035.
-
4. What are the key growth drivers of the France hernia repair devices market?The large number of patients being diagnosed with hernias, an increase in the older population at risk for developing hernias, and improved access to medical care through supportive healthcare systems.
-
5. Which product segment dominated the market in 2024?The hernia mesh segment dominated the market in 2024.
-
6. What segments are covered in the France hernia repair devices market report?The France hernia repair devices market is segmented on the basis of product, procedure type, and surgery type.
Need help to buy this report?