France Automated External Defibrillator Market Size, Share, By Product (Manual, Wearable Cardioverter Defibrillator, and Automated), By End Use (Pre-hospital, Public Access Market, Alternate Care Market, Home Healthcare, and Hospital), France Automated External Defibrillator Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareFrance Automated External Defibrillator Market Insights Forecasts to 2035
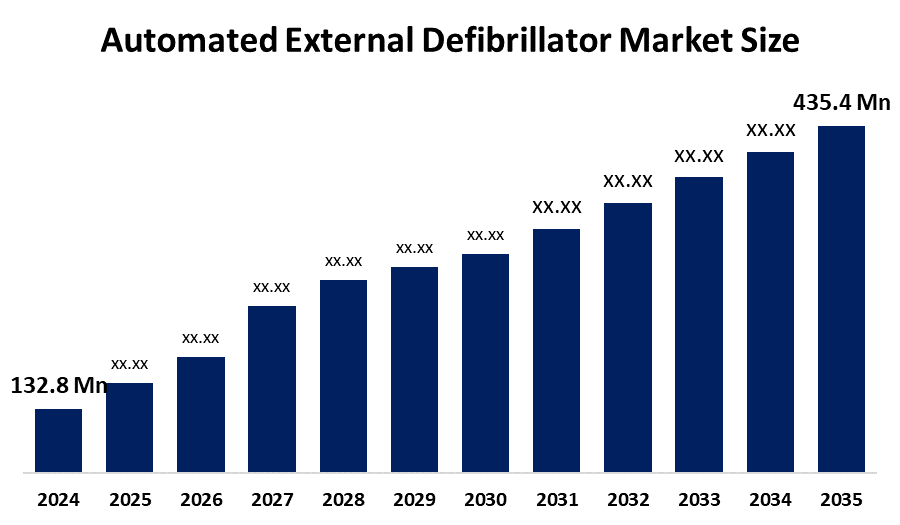
- France Automated External Defibrillator Market Size 2024: USD 132.8 Million
- France Automated External Defibrillator Market Size 2035: USD 435.4 Million
- France Automated External Defibrillator Market CAGR 2024: 11.4%
- France Automated External Defibrillator Market Segments: Product and End Use
Get more details on this report -
The France Automated External Defibrillator Market consists of manufacturing and selling portable devices which deliver electric shocks to restore normal heart rhythms during sudden cardiac arrest events that occur in hospitals, ambulances, airports, schools and various public locations.
The France automated external defibrillator market holds strong growth potential because of expanding public access defibrillation laws, increasing cardiac arrest awareness, government healthcare investments and smart connected AED adoption and growing deployment of defibrillators in airports, schools, shopping malls and office buildings.
France Automated External Defibrillator Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 132.8 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 11.4% |
| 2035 Value Projection: | USD 435.4 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 195 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Product, By End Use |
| Companies covered:: | Philips Healthcare, Stryker Corporation, ZOLL Medical Corporation, Schiller Médical, Safe Life, Nihon Kohden France, Mindray Medical France, Citycare, Defibtech, CU Medical Systems, Bexen Cardio, Others |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Market Dynamics of the France Automated External Defibrillator Market:
The France automated external defibrillator market is driven by rising cardiovascular disease prevalence, strong government regulations mandating AED installation in public places, increasing awareness of sudden cardiac arrest, and well-developed emergency medical services. Growth is further supported by workplace safety norms, expansion of public access defibrillation programs, and technological advancements such as connected, easy-to-use, and lightweight AED devices.
The France automated external defibrillator market is restrained by the high device and maintenance costs, limited public training, periodic battery and electrode replacement expenses, and budget constraints in small institutions or rural healthcare facilities.
The future of the France automated external defibrillator market is bright and promising, with expanding smart AED adoption, digital monitoring integration, stricter public safety regulations, and increasing installations across schools, transport hubs, corporate offices, and community centers.
Market Segmentation
The France automated external defibrillator market share is classified into products and end uses.
By Product:
The France automated external defibrillator market is divided by product into manual, wearable cardioverter defibrillator, and automated. Among these, the automated segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. Because the system is being increasingly used in public places, people need emergency response services that can reach them outside of hospitals.
By End Use:
The France automated external defibrillator market is divided by end use into pre-hospital, public access market, alternate care market, home healthcare, and hospital. Among these, the hospital segment accounted for the largest market share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. Because of increased patient traffic and to meet emergency preparedness requirements while maintaining essential defibrillator capabilities in both its critical care and emergency departments.
Competitive Analysis:
The report offers the appropriate analysis of the key organisations/companies involved within the France automated external defibrillator market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
Top Key Companies in France Automated External Defibrillator Market:
- Philips Healthcare
- Stryker Corporation
- ZOLL Medical Corporation
- Schiller Médical
- Safe Life
- Nihon Kohden France
- Mindray Medical France
- Citycare
- Defibtech
- CU Medical Systems
- Bexen Cardio
- Others
Key Target Audience
- Market Players
- Investors
- End Users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the France, regional, and country levels from 2020 to 2035. Sperical insights has segmented the France automated external defibrillator market based on the below-mentioned segments
France Automated External Defibrillator Market, By Product
- Manual
- Wearable Cardioverter Defibrillator
- Automated
France Automated External Defibrillator Market, By End Use
- Pre hospital
- Public Access market
- Alternate care market
- Home healthcare
- Hospital
Frequently Asked Questions (FAQ)
-
Q: What is the France automated external defibrillator market size?A: France automated external defibrillator market is expected to grow from USD 132.8 million in 2024 to USD 435.4 million by 2035, growing at a CAGR of 11.4% during the forecast period 2025-2035.
-
Q: What are the key growth drivers of the market?A: the rising cardiovascular disease prevalence, strong government regulations mandating AED installation in public places, increasing awareness of sudden cardiac arrest, and well-developed emergency medical services. Growth is further supported by workplace safety norms, expansion of public access defibrillation programs, and technological advancements such as connected, easy-to-use, and lightweight AED devices.
-
Q: What factors restrain the France automated external defibrillator market?A: Constraints include the high device and maintenance costs, limited public training, periodic battery and electrode replacement expenses, and budget constraints in small institutions or rural healthcare facilities.
-
Q: How is the market segmented by product?A: The market is segmented into manual, wearable cardioverter defibrillator, and automated.
-
Q: Who are the key players in the France automated external defibrillator market?A: Key companies include Philips Healthcare, Stryker Corporation, ZOLL Medical Corporation, Schiller Medical, Safe Life, Nihon Kohden (Nihon Kohden France), Mindray (Mindray Medical France), Citycare, Defibtech, CU Medical Systems, Bexen Cardio, and Others.
-
Q: Who are the target audiences for this market report?A: The report targets market players, investors, End Users, government authorities, consulting and research firms, venture capitalists, and value-added resellers (VARs).
-
Q: What is the France automated external defibrillator market size?A: France automated external defibrillator market is expected to grow from USD 132.8 million in 2024 to USD 435.4 million by 2035, growing at a CAGR of 11.4% during the forecast period 2025-2035.
-
Q: What are the key growth drivers of the market?A: the rising cardiovascular disease prevalence, strong government regulations mandating AED installation in public places, increasing awareness of sudden cardiac arrest, and well-developed emergency medical services. Growth is further supported by workplace safety norms, expansion of public access defibrillation programs, and technological advancements such as connected, easy-to-use, and lightweight AED devices.
-
Q: What factors restrain the France automated external defibrillator market?A: Constraints include the high device and maintenance costs, limited public training, periodic battery and electrode replacement expenses, and budget constraints in small institutions or rural healthcare facilities.
-
Q: How is the market segmented by product?A: The market is segmented into manual, wearable cardioverter defibrillator, and automated.
-
Q: Who are the key players in the France automated external defibrillator market?A: Key companies include Philips Healthcare, Stryker Corporation, ZOLL Medical Corporation, Schiller Medical, Safe Life, Nihon Kohden (Nihon Kohden France), Mindray (Mindray Medical France), Citycare, Defibtech, CU Medical Systems, Bexen Cardio, and Others.
-
Q: Who are the target audiences for this market report?A: The report targets market players, investors, End Users, government authorities, consulting and research firms, venture capitalists, and value-added resellers (VARs).
Need help to buy this report?