Global Force Sensing Ablation Catheters Market Size, Share, and COVID-19 Impact Analysis, by Product Type (Irrigated and Non-Irrigated Force Sensing Ablation Catheters), by Application (Atrial Fibrillation, Ventricular Tachycardia, Supraventricular Tachycardia, and Others), by End User (Hospitals, Specialty Clinics, Ambulatory Surgical Centers, and Others), and by Region (North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa) — Analysis and Forecast, 2025-2035
Industry: HealthcareGlobal Force Sensing Ablation Catheters Market Insights Forecasts to 2035
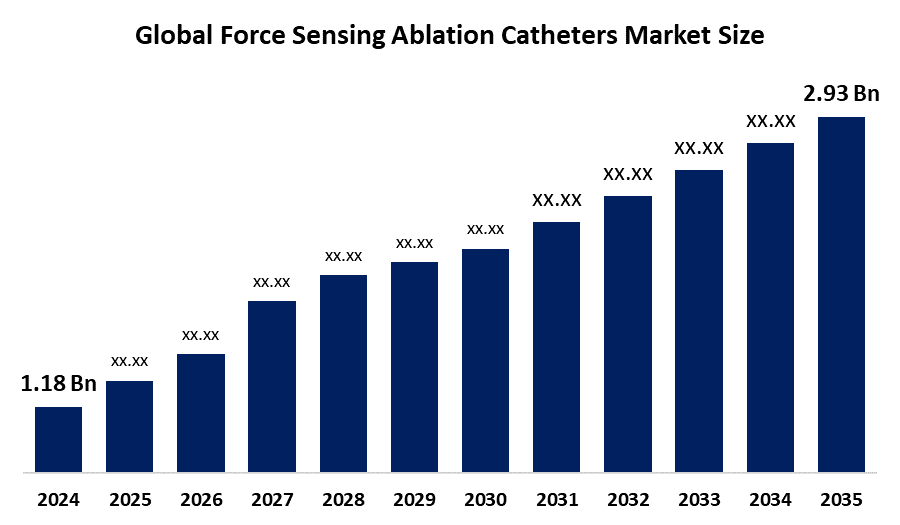
- The Global Force Sensing Ablation Catheters Market Size Was Estimated at USD 1.18 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of around 8.62% from 2025 to 2035
- The Worldwide Force Sensing Ablation Catheters Market Size is Expected to Reach USD 2.93 Billion by 2035
- Asia Pacific is expected to grow the fastest during the forecast period.
Get more details on this report -
According to a research report published by Spherical Insights and Consulting, the global Force Sensing Ablation Catheters Market size was worth around USD 1.18 Billion in 2024 and is predicted to grow to around USD 2.93 Billion by 2035 with a compound annual growth rate (CAGR) of 8.62% from 2025 and 2035. The market for pressure ablation catheters has several opportunities for growth due to advancements in techniques and technologies that prove superiority over anti-arrhythmic drugs.
Market Overview
The Global Pressure Ablation Catheters Market Size industry encompasses the catheters that use physical pressure or energy to destroy small amounts of heart tissue to correct irregular heart rhythms (arrhythmias). Pressure ablation catheters aid in delivering controlled energy to cardiac tissue for creating lesions, preventing abnormal electrical pathways. The increasing global prevalence of cardiac diseases, especially cardiac arrhythmias like atrial fibrillation, is driving the demand for pressure ablation catheters. Atrial fibrillation is the most frequent cardiac arrhythmia, which is estimated to affect 6-12 million people in the US by 2050, and 17.9 million people in Europe by 2060. Further, at the global pace, future projections suggested absolute atrial fibrillation burden may increase by >60% in 2050.
Innovation and market expansion are anticipated as a result of major players' growing R&D expenditures. For instance, science partner Journals published a research article on ‘Multifunctional Magnetic Catheter Robot with Triaxial Force Sensing Capability for Minimally Invasive Surgery’ which was supported by National Key Research and Development Project under Grant 2023YFB4705300, and other foundations and laboratory institutes. The development of novel technologies for improving safe and effective lesion delivery is anticipated to drive a huge surge in the global force sensing ablation catheters market. For instance, the SmartTouch catheter is an open irrigated tip catheter integrated within the CARTO 3 3D mapping system.
Report Coverage
This research report categorizes the force sensing ablation catheters market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the force sensing ablation catheters market. Recent market developments and competitive strategies such as expansion, type launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the force sensing ablation catheters market.
Global Force Sensing Ablation Catheters Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 1.18 Billion |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR of 8.62% |
| 2035 Value Projection: | USD 2.93 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 240 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Product Type, By Application |
| Companies covered:: | Abbott Laboratories, Boston Scientific Corporation, Biosense Webster (Johnson & Johnson), Medtronic plc, Biotronik SE & Co. KG, MicroPort Scientific Corporation, Acutus Medical, Inc., Lepu Medical Technology (Beijing) Co., Ltd., Osypka AG, CathRx Ltd., Imricor Medical Systems, Inc., Shanghai MicroPort EP MedTech Co., Ltd., Stereotaxis, Inc., Baylis Medical Company Inc., Japan Lifeline Co., Ltd., and other key players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The market size for force sensing ablation catheters is driven by the evolving healthcare sector, along with its use in a wide variety of applications, including atrial fibrillation, ventricular tachycardia, superventricular tachycardia, and other arrhythmias. The development of advanced solutions in ablation catheter by major manufacturers is propelling the market growth. For instance, in May 2025, Abbott introduced the TactiFlex sensor-enabled ablation catheter, which is the world’s first ablation catheter with a flexible tip and contact force technology. Additionally, the increasing ageing population is contributing to the market demand. Atrial fibrillation is the most common arrhythmia encountered in clinical practice, accounting for more than 2 million adults in the United States.
Restraining Factors
The force sensing ablation catheters market is restricted by the increased cost of advanced ablation technologies and limited reimbursement, which restricts its adoption, especially in the low and middle-class regions. Further, regulatory challenges and potential risk factors, including cardiac perforation or thromboembolic events, are hindering the market growth.
Market Segmentation
The force sensing ablation catheters market share is classified into product type, application, and end user.
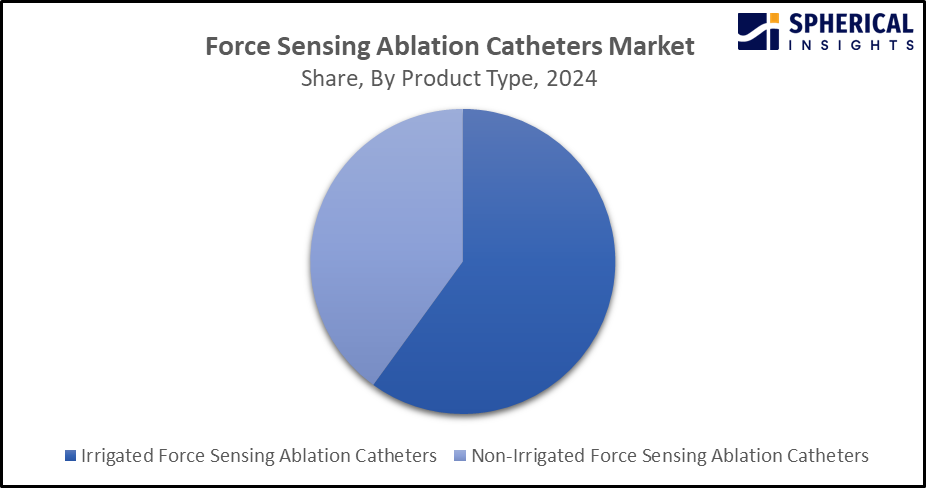
- The irrigated force sensing ablation catheters segment dominated the market with a substantial share in 2024 and is projected to grow at a substantial CAGR of nearly 10-13% during the forecast period.
Based on the product type, the force sensing ablation catheters market is divided into irrigated force sensing ablation catheters and non-irrigated force sensing ablation catheters. Among these, the irrigated force sensing ablation catheters segment dominated the market with a substantial share in 2024 and is projected to grow at a substantial CAGR of nearly 10-13% during the forecast period. There is an ongoing study on the use of an irrigated contact force-sensing catheter for redo ablation of slow-fast atrioventricular nodal reentrant tachycardia in pediatric and adolescent patients. The superior performance of irrigated force-sensing ablation catheters for managing complex arrhythmias is responsible for driving the market demand. Further, integration of innovative solutions like multi-electrode and steerable catheters for enhancing device versatility and effectiveness is propelling the market growth.
Get more details on this report -
- The atrial fibrillation segment accounted for the largest revenue share of over 60-70% in 2024 and is anticipated to grow at a significant CAGR during the forecast period.
Based on the application, the force sensing ablation catheters market is divided into atrial fibrillation, ventricular tachycardia, supraventricular tachycardia, and others. Among these, the atrial fibrillation segment accounted for the largest revenue share of over 60-70% in 2024 and is anticipated to grow at a significant CAGR during the forecast period. Contact force sensing radiofrequency catheter ablation therapy aids in achieving electrical isolation of the pulmonary veins, which is the cornerstone of treating atrial fibrillation. An increasing global prevalence of atrial fibrillation, along with the adoption of minimally invasive ablation procedures, is driving the segmental market growth.
- The hospitals segment accounted for the largest market revenue share of 36.8-81.5% in 2024 and is anticipated to grow at a significant CAGR during the forecast period.
Based on the end user, the force sensing ablation catheters market is divided into hospitals, specialty clinics, ambulatory surgical centers, and others. Among these, the hospitals segment accounted for the largest market revenue share of 36.8-81.5% in 2024 and is anticipated to grow at a significant CAGR during the forecast period. It was estimated that over six million people in the US live with AF, which is projected to increase 2.5-fold by 2050, and each year, over 454,000 hospitalizations are attributed to AF. The presence of advanced electrophysiology labs, skilled healthcare professionals, and comprehensive cardiac care facilities is responsible for propelling the market growth in the hospitals segment.
Regional Segment Analysis of the Force Sensing Ablation Catheters Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
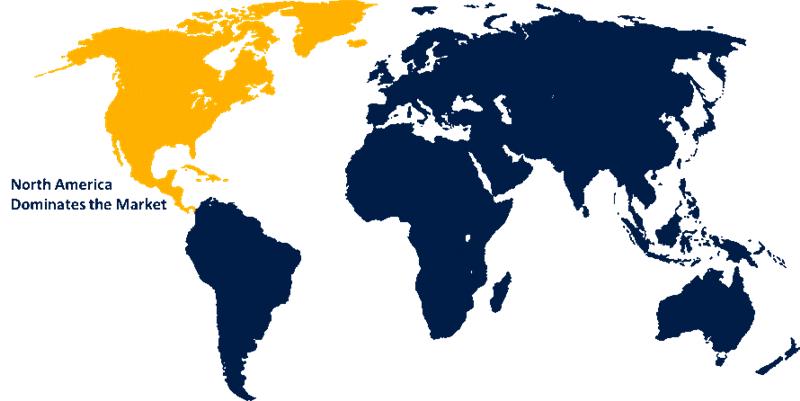
North America is anticipated to hold the largest share of the force sensing ablation catheters market over the predicted timeframe.
North America is anticipated to hold the largest share of approximately 43.5% to 48.5% in the force sensing ablation catheters market over the predicted timeframe. The market ecosystem in North America is strong, due to cutting-edge startups. For instance, in January 2025, APT Medical’s groundbreaking EP PFA portfolio, which includes force-sensing PFA ablation catheter products, received NMPA approval. The market for pressure ablation catheters has been driven by the region's increasing prevalence of arrhythmia patterns and an increasing ageing population. The U.S. is dominating the North America market, ranging 65-85% share during the forecast period. This is attributed to the increasing technological advancements, along with an increasing prevalence of chronic diseases, including cardiovascular disorders.
Get more details on this report -
Asia Pacific is expected to grow at a rapid CAGR of about 12.1% in the force sensing ablation catheters market during the forecast period. The Asia Pacific area has a thriving market for pressure ablation catheters due to its region's increasing cancer and cardiac ailments prevalence rate and technological advancements for designing high-end products. The launch of new technologies for improving cardiac ablation procedures for AFib patients is propelling the market growth. For instance, in May 2025, Abbott launched the TectiFlex sensor-enabled ablation catheter for atrial fibrillation treatment. Japan is the leading country in the Asia Pacific force sensing ablation catheters market, with an estimated 18-22% share, due to an increasing ageing population, which is driving the need for treatment addressing cardiac arrhythmias.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the force sensing ablation catheters market, along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Abbott Laboratories
- Boston Scientific Corporation
- Biosense Webster (Johnson & Johnson)
- Medtronic plc
- Biotronik SE & Co. KG
- MicroPort Scientific Corporation
- Acutus Medical, Inc.
- Lepu Medical Technology (Beijing) Co., Ltd.
- Osypka AG
- CathRx Ltd.
- Imricor Medical Systems, Inc.
- Shanghai MicroPort EP MedTech Co., Ltd.
- Stereotaxis, Inc.
- Baylis Medical Company Inc.
- Japan Lifeline Co., Ltd.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In September 2025, Abbott India Limited, a prominent player in the Indian pharmaceutical sector, is exhibiting a robust financial trajectory and a promising valuation outlook. Abbott India Limited has consistently delivered strong financial results, reinforcing its market leadership.
- In January 2025, Johnson & Johnson MedTech, a global leader in cardiac arrhythmia treatment, announced European CE mark approval of the Dual Energy THERMOCOOL SMARTTOUCH SF Catheter for the treatment of cardiac arrhythmias.
- In December 2024, CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for cardiac arrhythmias, announced the first series of patients treated with investigational OptiShot Pulsed Field Ablation (PFA) System for the treatment of paroxysmal atrial fibrillation as part of the VISION AF clinical trial.
- In July 2023, Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & Johnson MedTech, announced that enrollment is complete in the SmartfIRE study designed to evaluate the safety and efficacy of its investigational THERMOCOOL SMARTTOUCH SF Dual Energy Catheter and investigational TRUPULSE Generator for the treatment of drug refractory symptomatic paroxysmal atrial fibrillation (AFib) during standard electrophysiology mapping and ablation procedures.
- In June 2023, the U.S. Food and Drug Administration (FDA) cleared Abbott’s TactiFlex Ablation Catheter, Sensor Enabled, which is the world's first ablation catheter with a flexible tip and contact force technology. The radiofrequency (RF) catheter has been approved to treat atrial fibrillation (AFib).
- In February 2023, Abbott announced two approvals as part of its growing suite of electrophysiology products in the global market. The company’s TactiFlex Ablation Catheter, Sensor Enabled, the world’s only ablation catheter with a flexible tip and contact force sensing, received CE Mark1 for treating people with abnormal heart rhythms like atrial fibrillation (AFib).
- In October 2022, Acutus Medical, Inc., an arrhythmia management company focused on improving the way cardiac arrhythmias are diagnosed and treated announced submission of pivotal clinical data from the AcQForce Flutter trial designed to gain marketing approval from the U.S. Food & Drug Administration (FDA) for the AcQBlate Force Sensing Ablation Catheter and System (AcQBlate FORCE).
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the force sensing ablation catheters market based on the below-mentioned segments:
Global Force Sensing Ablation Catheters Market, By Product Type
- Irrigated Force Sensing Ablation Catheters
- Non-Irrigated Force Sensing Ablation Catheters
Global Force Sensing Ablation Catheters Market, By Application
- Atrial Fibrillation
- Ventricular Tachycardia
- Supraventricular Tachycardia
- Others
Global Force Sensing Ablation Catheters Market, By End User
- Hospitals
- Specialty Clinics
- Ambulatory Surgical Centers
- Others
Global Force Sensing Ablation Catheters Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the market size of the force sensing ablation catheters market?The global force sensing ablation catheters market size is expected to grow from USD 1.18 Billion in 2024 to USD 2.93 Billion by 2035, at a CAGR of 8.62% during the forecast period 2025-2035.
-
2. Which region holds the largest share of the force sensing ablation catheters market?North America is anticipated to hold the largest share of the force sensing ablation catheters market over the predicted timeframe.
-
3. What is the forecasted CAGR of the Global Force Sensing Ablation Catheters Market from 2024 to 2035?The market is expected to grow at a CAGR of around 8.62% during the period 2024–2035.
-
4. Who are the top companies operating in the Global Force Sensing Ablation Catheters Market?Key players include Abbott Laboratories, Boston Scientific Corporation, Biosense Webster (Johnson & Johnson), Medtronic plc, Biotronik SE & Co. KG, MicroPort Scientific Corporation, Acutus Medical, Inc., Lepu Medical Technology (Beijing) Co., Ltd., Osypka AG, CathRx Ltd., Imricor Medical Systems, Inc., Shanghai MicroPort EP MedTech Co., Ltd., Stereotaxis, Inc., Baylis Medical Company Inc., and Japan Lifeline Co., Ltd.
-
5. Can you provide company profiles for the leading force sensing ablation catheters manufacturers?Yes. For example, Abbott Laboratories is an American multinational medical devices and health care company with a significant presence in the medical device market, especially in electrophysiology and cardiac ablation technologies. Boston Scientific Corporation is a worldwide developer, manufacturer and marketer of medical devices that are used in a broad range of interventional medical specialities, including interventional cardiology.
-
6. What are the main drivers of growth in the force sensing ablation catheters market?The development of advanced solutions in ablation catheters, evolving healthcare sectors, and increasing ageing population are major market growth drivers of the force sensing ablation catheters market.
-
7. What challenges are limiting the force sensing ablation catheters market?An increased cost of advanced ablation technologies and limited reimbursement, as well as regulatory challenges and potential risk factors, remain key restraints in the force sensing ablation catheters market.
Need help to buy this report?