Europe Viral Vector Market Size, Share, and COVID-19 Impact Analysis, By Vector Type (Adeno-Associated Viruses, Lentivirus, Retrovirus, Adenovirus and Others), By Application (Cell and Gene Therapy, Vaccine, and Biopharmaceutical and Pharmaceuticals Discovery), By End User (Pharmaceutical and Biotechnology Companies, Academic and Research Institutes, CROs and CMOs) , and Europe Viral Vector Market Insights, Industry Trends, Forecast to 2035
Industry: HealthcareEurope Viral Vector Market Insights Forecasts to 2035
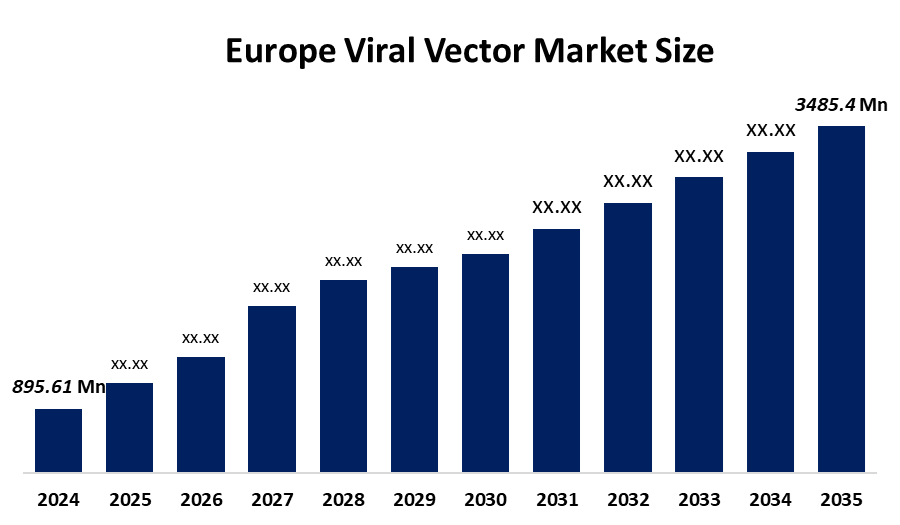
- The Europe Viral Vector Market Size Was Estimated at USD 895.61 Million in 2024
- The Market Size is Expected to Grow at a CAGR of Around 13.15% from 2025 to 2035
- The Europe Viral Vector Market Size is Expected to Reach USD 3485.4 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, The Europe viral vector Market Size is anticipated to reach USD 3485.4 Million by 2035, growing at a CAGR of 13.15% from 2025 to 2035. The market is driven by the demand for gene therapy is increasing due to awareness about the potential benefits in the sphere of healthcare professionals and patients.
Market Overview
A viral vector is a virus that has been modified, made harmless and is used as a delivery vehicle to introduce the genetic material to the target cells. Researchers develop these tools by removing the virus's disease-causing and replication genes, substituting them with a particular payload such as a therapeutic gene or instructions for an antigen. Scientists are increasingly relying on viral vectors in experiments as a way to get genes into cells. It is regarded as the best technique for transferring genes, altering a specific cell type or tissue and controlling them to create therapeutic proteins.
In September 2024, Rentschler Biopharma, a contract development and manufacturing organization (CDMO) for biopharmaceuticals, declared the opening of an expanded service offering at its advanced therapies facility in Stevenage, UK. Europe in 2023, where the overall investment in gene therapy is expected to be USD 1.2 billion in the year.
The European firms are rapid in absorbing modern technologies such as bioprocessing, automation, and analytical tools. The production processes of viral vectors are enhanced in terms of efficiency and quality by technological improvements. The European Pharmacopoeia Commission (EPC) is specifying new standard methods for Gene Therapy Medicinal Products for human application. Regulatory health authorities, for instance, the EMA, are always keeping an eye on the safety of viral vector products.
Report Coverage
This research report categorizes the market for the Europe viral vector market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Europe viral vector market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Europe viral vector market.
Europe Viral Vector Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 895.61 Million |
| Forecast Period: | 2024-2035 |
| Forecast Period CAGR 2024-2035 : | CAGR of 13.15% |
| 2035 Value Projection: | USD 3485.4 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 240 |
| Tables, Charts & Figures: | 90 |
| Segments covered: | By Vector |
| Companies covered:: | Lonza, Merck KGaA, Oxford Biomedica, Novartis, Sartorius AG, Thermo Fisher Scientific, SIRION Biotech, Plasmid Factory, Charles River Laboratory, Roche, and Other key players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The viral vector market in Europe is driven by the increasing cancer patient population in Europe, the increase in gene therapy research and development activities, and the significant funding for research and development activities in the gene therapy area provided by the European governments. The steep rise in the need for advanced gene and cell therapy solutions, next-generation vaccines, and state-of-the-art genetic research. Viral vectors are being used more frequently in laboratories to introduce genetic material into cells. The advancements in bioprocessing, automation, and analytical tools are making the production of viral vectors more efficient, higher in quality, and larger in scale.
Restraining Factors
The viral vector market in Europe is restrained by the viral vector and plasmid DNA production being highly technologically advanced. The lack of skilled labor, long production times for batches, and high rates of process failure are all hurdles. The high cost of production limits the growth of the market. The production cycle is dependent on a reliable and continuous supply of certain specialized raw materials, one of which is high-quality plasmid DNA.
Market Segmentation
The Europe viral vector market share is categorised into vector type, application and end user.
- The adeno-associated viruses segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Europe viral vector market is segmented by vector type into adeno-associated viruses, lentivirus, retrovirus, adenovirus and others. Among these, the adeno-associated viruses segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The growth of the segment is driven by its advantages concerning safety, efficiency in non-dividing cells, and application in already approved in vivo therapies, which were regarded as the main characteristics of the vector. It possessed an excellent safety profile (low immune response), long-term gene expression, and efficient delivery to non-dividing cells (neurons, eye cells), and in fact, it was used in many approved gene therapies.
- The cell and gene therapy segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period.
Based on application, the Europe viral vector market is segmented into cell and gene therapy, vaccine, and biopharmaceutical and pharmaceutical discovery. Among these, the cell and gene therapy segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period. The segmental growth is driven by the rising number of clinical approvals and demand for personalized medicines is not the only cause behind the use of viral vaccines such as Adenovirus vectors which have also become very large, supported by the needs of infectious diseases, the extensive clinical use and the rise in approval of therapies for rare diseases, with the vectors playing an important role as the carriers of therapeutic genes.
- The pharmaceutical and biotechnology companies segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Europe viral vector market is segmented by end user into pharmaceutical and biotechnology companies, academic and research institutes, CROs and CMOs. Among these, the pharmaceutical and biotechnology companies segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. The growth of the segment is driven by the investment in gene therapy research and development for treating cancer and favorable regulations. High R&D spending, a large number of gene therapy programs, and a favorable regulatory environment are the main drivers of their market position.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Europe viral vector market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companie
- Lonza
- Merck KGaA
- Oxford Biomedica
- Novartis
- Sartorius AG
- Thermo Fisher Scientific
- SIRION Biotech
- Plasmid Factory
- Charles River Laboratory
- Roche
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
In November 2025, AAVantgarde closed a Series B financing round, and AGC Biologics announced a new manufacturing agreement with the biotechnology company, marking AGC Biologics’ latest advancement in the adeno-associated virus market.
In June 2025, ArcticZymes Technologies ASA is pleased to announce the expansion of its GMP product range, with the introduction of M-SAN HQ GMP, a new GMP-grade nuclease specifically designed for viral vector manufacturing.
In February 2025, in Slovenia, Novartis opens its first specialized viral vector production facility in Europe, bringing new technology to Slovenia to support the production of breakthrough cell and gene therapies.
Market Segment
This study forecasts revenue at the Europe, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Europe viral vector market based on the below-mentioned segments:
Europe Viral Vector Market, By Vector Type
- Adeno-Associated Viruses
- Lentivirus
- Retrovirus
- Adenovirus
- Others
Europe Viral Vector Market, By Application
- Cell and Gene Therapy
- Vaccine
- Biopharmaceutical and Pharmaceuticals Discovery
Europe Viral Vector Market, By End User
- Pharmaceutical and Biotechnology Companies
- Academic and Research Institutes
CROs and CMOs
Frequently Asked Questions (FAQ)
-
What is the Europe viral vector market size?Europe viral vector market size is expected to grow from USD 895.61 million in 2024 to USD 3485.4 million by 2035, growing at a CAGR of 13.15% during the forecast period 2025-2035
-
What is viral vector, and its primary use?A viral vector is a virus that has been modified, made harmless and is used as a delivery vehicle to introduce the genetic material to the target cells. Researchers develop these tools by removing the virus's disease-causing and replication genes, substituting them with a particular "payload" such as a therapeutic gene or instructions for an antigen
-
What are the key growth drivers of the market?Market growth is driven by the increasing cancer patient population in Europe, the increase in gene therapy research and development activities, and the significant funding for research and development activities in the gene therapy area provided by the European governments. The steep rise in the need for advanced gene and cell therapy solutions, next-generation vaccines, and state-of-the-art genetic research
-
What factors restrain the Europe viral vector market?The market is restrained by the viral vector and plasmid DNA production being highly technologically advanced and also very expensive. The lack of skilled labor, long production times for batches, and high rates of process failure are all hurdles
-
How is the market segmented by vector type?The market is segmented into adeno-associated viruses, lentivirus, retrovirus, adenovirus and others
-
Who are the key players in the Europe viral vector market?Key companies include Lonza, Merck KGaA, Oxford Biomedica, Novartis, Sartorius AG, Thermo Fisher Scientific, SIRION Biotech, Plasmid Factory, Charles River Laboratory, and Roche.
Need help to buy this report?