Global Dengue Testing Market Size, Share and COVID-19 Impact Analysis, By Product Scope (ELISA based Tests, Dengue IgG/IgM Detection Kits, RT PCR Tests, Rapid Diagnostic Tests (RDTs), NS1 Antigen Detection Kits and Lateral Flow Immunoassay), By End Use (Clinical Labs, Hospitals/Clinics and Home Healthcare), By Region (North America, Europe, Asia Pacific, Latin America, Middle East and Africa), Analysis and Forecast 2025 to 2035
Industry: HealthcareGlobal Dengue Testing Market Insights Forecasts to 2035
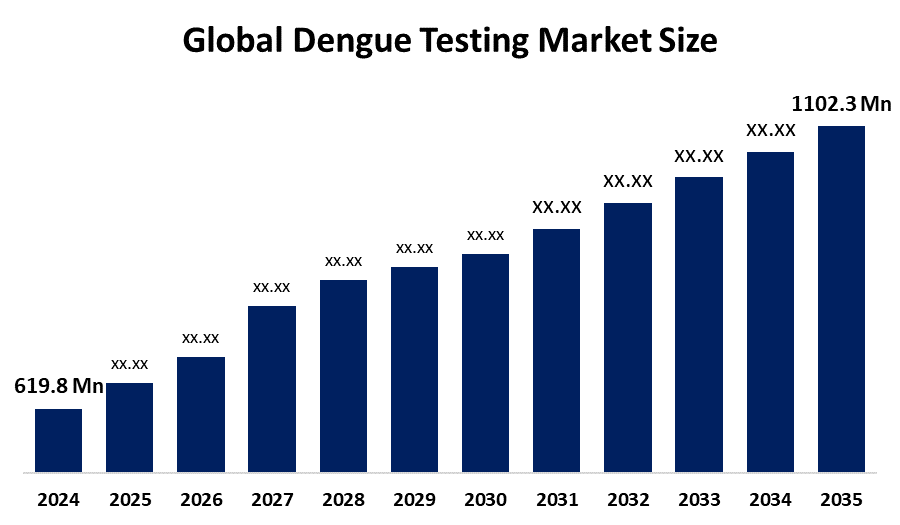
- The Global Dengue Testing Market Size Was Estimated at USD 619.8 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 5.37% from 2025 to 2035
- The Worldwide Dengue Testing Market Size is Expected to Reach USD 1102.3 Million by 2035
- Asia Pacific is expected to grow the fastest during the forecast period.
Get more details on this report -
According to a research report published by Spherical Insights and Consulting, the global Dengue Testing Market Size was worth around USD 619.8 Million in 2024, growing to 652.7 Million in 2025, and is predicted to grow to around USD 1102.3 Million by 2035 with a compound annual growth rate (CAGR) of 5.37% from 2025 to 2035. Increased public health activities, technical improvements, and the growing global dengue prevalence are driving the dengue diagnostic market. The major dengue epidemic in Latin America in 2024 brought to light the pressing need for precise diagnostics, which led to government financing and awareness initiatives to enhance early identification and outbreak control, particularly in endemic areas.
Global Dengue Testing Market Forecast and Revenue Size
- 2024 Market Size: USD 619.8 Million
- 2025 Market Size: USD 652.7 Million
- 2035 Projected Market Size: USD 1102.3 Million
- CAGR (2025-2035): 5.37%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
Market Overview
The dengue testing market includes diagnostic instruments and methods for identifying dengue infections, which are fuelled by an increase in cases, technical developments, and global public health campaigns. To enhance early diagnosis and management of the disease through improved detection of dengue, companies like Abbott Laboratories and Roche Diagnostics have begun incorporating rapid testing and multiplex PCR assays. While ELISA remains the clinical gold standard for detecting dengue antibody and NS1 antigen to discriminate among the four viral serotypes, innovations like AccoBiotech’s 2025 AccoDengue Home Test enable at-home detection in a cost-effective and user-friendly manner, crucial in resource-limited settings. Point-of-care testing (POCT) tools, like Mylab Discovery Solutions’ multiplex RT-PCR kit, offer speed and accuracy of diagnosis in decentralised settings for the immediate management of co-infections. The implementation of these newly developed tools, especially in low- and middle-income countries where access to laboratories might not be possible, is critical for improved patient care. Government initiatives are strengthening detection networks and epidemic tracking, such as the National Vector Borne Disease Control Program in India and public health campaigns in Latin America. The dengue testing market is growing quickly because of public health measures and technology innovation, which also improve early detection, patient outcomes, and outbreak control around the world. The dengue testing market is led by innovations in fast and molecular diagnostics, such as RT-PCR and CRISPR tests. Mergers and acquisitions of companies increase access to low-cost diagnostic tools and the development of technology, particularly in Asia and Latin America.
Why does the United States lead the North American dengue testing market?
Increasing dengue instances among travellers, combined with the CDC's recommendations for dengue testing, are expanding the dengue testing market in the US. To prospective testing options, clinical laboratories and point of care (POC) centres are adding multiplex RT-PCR testing and the NS1 antigen to infectious disease panels, while insurance reimbursement has made access to dengue testing easier. Early detection efforts are fueled by border and airport public health initiatives and make the US the primary market.
Asia Pacific Dengue Testing Market Trends
How is the Asia Pacific region expected to be the fastest-growing dengue testing market with the fastest growth rate?
Key Market Insights
- North America is expected to account for the largest share in the Dengue Testing market during the forecast period.
- In terms of product scope, the ELISA-based tests segment is projected to lead the Dengue Testing market throughout the forecast period
- In terms of end use, the clinical labs segment captured the largest portion of the market
Dengue Testing Market Trends
- Rapid diagnostic tests (RDTs) are being used more often for point-of-care, early detection.
- Accuracy is increased by developments in molecular diagnostics such as RT-PCR and CRISPR-based techniques.
- Creation of multiplex assays to differentiate dengue from related illnesses like chikungunya and Zika.
- Expanding the number of home-based testing options to improve accessibility in environments with limited resources.
- Increasing acquisitions and mergers to broaden technology portfolios and simplify international distribution.
Report Coverage
This research report categorizes the dengue testing market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyzes the key growth drivers, opportunities, and challenges influencing the dengue testing market. Recent market developments and competitive strategies, such as expansion, type launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyzes their core competencies in each sub-segment of the dengue testing market.
Global Dengue Testing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 619.8 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 5.37% |
| 2035 Value Projection: | USD 1102.3 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 250 |
| Tables, Charts & Figures: | 119 |
| Segments covered: | By Product Scope, By End-use, By Regional Analysis |
| Companies covered:: | Thermo Fisher Scientific Inc., Abbott Laboratories, F. Hoffmann-La Roche Ltd., InBios International, Inc., NovaTec Immundiagnostica GmbH, Abnova Corporation, PerkinElmer Inc. (Euroimmun AG), Certest Biotec, DiaSorin S.p.A., Quest Diagnostics Incorporated, Other Notable Participants |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving factors: The need for vaccines is driven by rising dengue incidences in the population.
The increasing incidence of dengue fever around the world, especially in tropical and subtropical regions of Asia, Latin America and Africa, is facilitating intensive growth of the dengue testing market. The expansion of viral diseases has been influenced by factors including urbanisation, climate change, and insufficient mosquito control. There is a rising demand for rapid and accurate diagnostics such as antigen tests, molecular tests, and serological assays. Due to emerging market technologies and spending in the healthcare arena, there is a greater demand for point-of-care and home testing kits to help manage subsequent outbreaks.
Restraining Factor: Market Efficiency is Limited by Difficulties in Diagnosing Severe Dengue
The incapacity of existing tests, such as NS1 antigen and IgM/IgG antibody assays, to reliably forecast disease severity is a significant obstacle in the dengue testing business. This frequently leads to needless hospital stays and overburdened medical facilities. Blood tests are expensive and time-consuming for tracking severe symptoms, and correct identification is made more difficult by overlapping symptoms in infections like chikungunya and leptospirosis.
Market Segmentation
The global dengue testing market is divided into product scope and end use.
Global Dengue Testing Market, By Product Scope:
Why does the ELISA-based tests segment hold the largest revenue share in the global dengue testing market, accounting for approximately 45.1% of the total market during the forecast period?
The ELISA-based tests segment led the dengue testing market, generating the largest revenue share. They have high sensitivity, specificity, and the ability to detect dengue antigens (NS1) and antibodies (IgM and IgG). These tests also offer flexible diagnostic windows and are particularly useful in both the early and later stages of disease. After the dramatic rise in dengue's global incidence in 2024, the need for accurate laboratory-based diagnosis has increased. Companies such as Abbott and Bio-Rad have added novel, faster multi-serotype detection kits to their ELISA test line-ups. In addition, ELISA is being increasingly adopted as an important strategy for large-scale dengue surveillance, given support from both governments and international health agencies for centralised disease monitoring.
The lateral flow immunoassay segment in the dengue testing market is expected to grow at the fastest CAGR over the forecast period. Its simplicity, quick outcomes, and little infrastructure needs. LFIAs are especially well-suited for point-of-care testing in situations with limited laboratory access that are remote and resource-constrained. The dengue outbreak in 2024 in Latin America and Southeast Asia highlighted the need for prompt diagnosis, and LFIA kits such as Abbott's SD BIOLINE Dengue Duo and Panbio Dengue Rapid Tests became quite popular.
Global Dengue Testing Market, By End Use:
Why do clinical labs represent the largest end use segment, capturing approximately 44.16% market share during the forecast period in the global dengue testing market?
The clinical labs segment held the largest market share in the dengue testing market. Growth is fuelled by higher investments in RT-PCR systems, high-throughput ELISA, and laboratory automation, which allow for same-day turnaround. While networks of private labs in Brazil and India provide multiplex testing during peak seasons, major businesses like LabCorp and Quest Diagnostics in North America have incorporated dengue RT-PCR into their infectious disease panels. Test volumes are also rising as a result of public health programs that are combining surveillance testing in local reference labs.
The hospitals /clinics segment in the dengue testing market is expected to grow at the fastest CAGR over the forecast period. The growing number of dengue cases that need prompt diagnosis and treatment, the availability of sophisticated diagnostic equipment, the expansion of the healthcare infrastructure, and the growing patient preference for expert medical care, the hospitals and clinics category is anticipated to develop at the quickest rate. The need for dengue testing in clinical and hospital settings is increased by these considerations.
Regional Segment Analysis of the Global Dengue Testing Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
North America Dengue Testing Market Trends
What factors contribute to the North America region holding the largest share of the global dengue testing market during the forecast period?
North America is dominant in the global dengue testing market share during the forecast period. Driven by increased use of point-of-care testing and strong public health surveillance. Investments in multiplex PCR platforms and quick NS1 antigen assays are mostly made in the United States and Canada. In order to ensure prompt outbreak response and patient management, federal programs support decentralised testing in clinics and urgent care centres, while increased importation of travel-related cases has prompted laboratory renovations.
Why does the United States lead the North American dengue testing market?
Increasing dengue instances among travellers, combined with the CDC's recommendations for dengue testing, are expanding the dengue testing market in the US. To prospective testing options, clinical laboratories and point of care (POC) centres are adding multiplex RT-PCR testing and the NS1 antigen to infectious disease panels, while insurance reimbursement has made access to dengue testing easier. Early detection efforts are fueled by border and airport public health initiatives and make the US the primary market.
Asia Pacific Dengue Testing Market Trends
How is the Asia Pacific region expected to be the fastest-growing dengue testing market with the fastest growth rate?
Asia Pacific is expected to be the fastest-growing dengue testing market due to several key factors. Since dengue is endemic in Thailand, Indonesia, and India, the Asia Pacific dengue testing industry has dominated the market. In regional labs, national vector control programs (like India's NVBDCP) provide funding for ELISA and RT-PCR, whereas community health centres use POC NS1 fast assays. Smartphone-connected devices allow for remote result reporting, and public-private collaborations increase the capacity for molecular testing. Additional factors driving market expansion in the region include urbanisation, climate change, and rising healthcare spending.
Why is Japan expected to register the highest CAGR in the dengue testing market during the forecast period?
The governmental funding of surveillance initiatives supports the extension of the use of automated ELISA and RT-PCR in public health laboratories for surveillance. Combined with this, multiplex tests differentiating dengue versus Zika and chikungunya are further innovations resulting from partnerships between academic institutions and biotech firms. In addition, there are rapid NS1 antigen tests also available at travel clinics at many major airports. Taken together with abundant healthcare funding and digital health platforms, these allow consistent expansion of dengue diagnostics.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global dengue testing market, along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
Key Players
- Thermo Fisher Scientific Inc.
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd.
- InBios International, Inc.
- NovaTec Immundiagnostica GmbH
- Abnova Corporation
- PerkinElmer Inc. (Euroimmun AG)
- Certest Biotec
- DiaSorin S.p.A.
- Quest Diagnostics Incorporated
- Other Notable Participants
Key Target Audience
- Market Participants
- Investors
- End Users
- Government Agencies and Regulators
- Consulting and Research Firms
- Venture Capitalists
- Value-Added Resellers (VARs)
Recent Developments
-
In December 2024, bioMérieux announced that its BIOFIRE FILMARRAY Tropical Fever (TF) Panel received Special 510(k) clearance from the U.S. Food and Drug Administration (FDA). This advanced polymerase chain reaction (PCR) testing solution enables rapid and precise identification of pathogens in patients with unexplained fever, including dengue, thereby supporting more effective and timely treatment decisions.
-
In September 2024, Mankind Pharma introduced RAPID NEWS self-test kits to provide convenient and reliable health solutions. These kits are designed for easy at-home use, allowing individuals to quickly test for dengue, urinary tract infections (UTIs), and menopause. By offering a private and accessible testing option, they help users detect health conditions early and seek timely medical intervention.
-
In June 2024, QIAGEN launched its new QIAcuity digital PCR assays for microbial applications, enhancing infectious disease research and surveillance by introducing assays for Dengue virus serotypes
-
In August 2023, Mylab Discovery Solutions introduced two new rapid point-of-care tests for dengue: the Rapid Gold Test and the high-accuracy Dry Luminescence Assay Test.
Market Segmentation
This report projects revenue at global, regional, and country levels from 2020 to 2035. Spherical Insights has categorized the dengue testing market into the following segments:
Global Dengue Testing Market, By Product Scope
- ELISA-based Tests,
- Dengue IgG/IgM Detection Kits
- RT-PCR Tests
- Rapid Diagnostic Tests (RDTs)
- NS1 Antigen Detection Kits
- Lateral Flow Immunoassay
Global Dengue Testing Market, By End-use
- Clinical Labs
- Hospitals /Clinics
- Home Healthcare
Global Dengue Testing Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the Dengue Testing market over the forecast period?The global Dengue Testing market is projected to expand at a CAGR of 5.37% during the forecast period.
-
2. What is the market size of the Dengue Testing market?The global Dengue Testing market size is expected to grow from USD 619.8 million in 2024 to USD 1102.3 million by 2035, at a CAGR 5.37% of during the forecast period 2025-2035.
-
3. Which region holds the largest share of the Dengue Testing market?North America is anticipated to hold the largest share of the Dengue Testing market over the predicted timeframe.
-
4. Who are the top 10 companies operating in the global Dengue Testing market?Thermo Fisher Scientific Inc., Abbott, F. Hoffmann‑La Roche Ltd., InBios International, Inc., NovaTec Immundiagnostica GmbH, Abnova Corporation, PerkinElmer Inc. (Euroimmun AG), Certest Biotec, DiaSorin S.p.A., Quest Diagnostics Incorporated, and Others.
-
5. What factors are driving the growth of the Dengue Testing market?Rising dengue incidence, technological advancements in diagnostics, increased public health awareness, government initiatives, and expanding healthcare infrastructure in endemic regions are key factors driving the growth of the dengue testing market.
-
6. What are the market trends in the Dengue Testing market?Rapid diagnostic tests (RDTs) are being used more often for point-of-care, early detection. Accuracy is increased by developments in molecular diagnostics such as RT-PCR and CRISPR-based techniques. Creation of multiplex assays to differentiate dengue from related illnesses like chikungunya and Zika, and expanding the number of home-based testing options to improve accessibility in environments with limited resources.
-
7. What are the main challenges restricting wider adoption of the Dengue Testing market?Wider adoption of dengue testing is hampered by a number of obstacles, such as restricted access in low-resource areas, the high expense of sophisticated diagnostic equipment, a lack of knowledge, inadequate insurance coverage, the inability to differentiate dengue from related infections, and logistical problems when distributing tests during outbreaks.
Need help to buy this report?