China In Vitro Diagnostics Market Size, Share, By Product (Instruments, Services), By Test Location (Point of Care, Home Care), China In Vitro Diagnostics Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareChina In Vitro Diagnostics Market Insights Forecasts to 2035
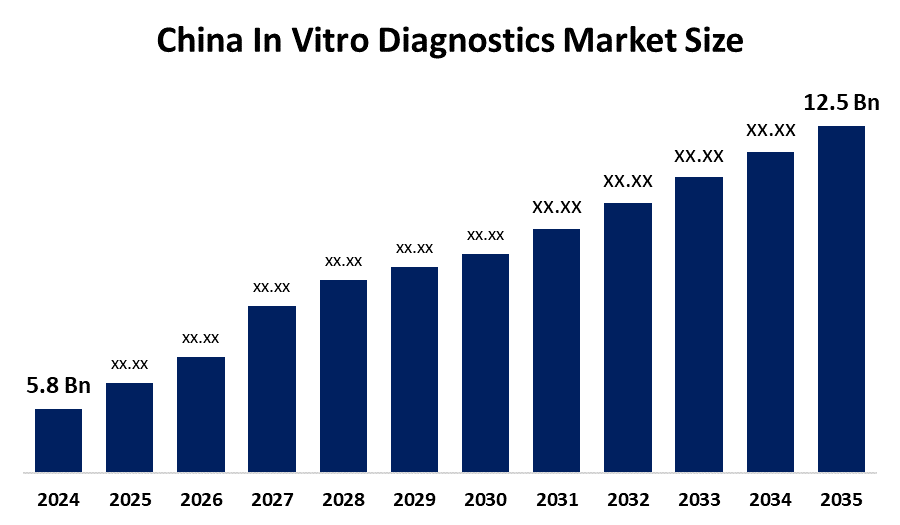
- China In Vitro Diagnostics Market Size 2024: USD 5.8 Bn
- China In Vitro Diagnostics Market Size 2035: USD 12.5 Bn
- China In Vitro Diagnostics Market CAGR 2024: 7.23%
- China In Vitro Diagnostics Market Segments: Product and Test Location
Get more details on this report -
The China in vitro diagnostics (IVD) market is a broad and the rapidly expanding sector encompassing clinical chemistry, immunoassays, molecular diagnostics, hematology, microbiology, and the point-of-care testing used for disease prevention, diagnosis, monitoring, and the health screening. The greater healthcare demand, an aging population, an increase in the prevalence of infectious and chronic diseases, greater health consciousness, and robust government backing for early diagnosis and healthcare modernization are now driving the industry. China is now one of the biggest and fastest-growing IVD markets in the world thanks to ongoing regulatory changes, improvements to hospitals and laboratories, advancements in molecular and genetic testing, and increased investments from both domestic inventors and international IVD businesses.
The Healthy China 2030 Initiative is a national strategy aimed at improving population health by shifting healthcare toward prevention, early diagnosis, and disease screening. This long-term focus increases demand for diagnostic testing of chronic and infectious diseases, directly supporting sustained growth of China’s in vitro diagnostics (IVD) market.
China’s in vitro diagnostics (IVD) market participants and clinical validation service providers which are increasingly leveraging digital pathology, automated laboratory systems, electronic data capture, cloud platforms, and the artificial intelligence. These tools enable real-time sample tracking, remote data collection from hospitals and laboratories, high-throughput testing, and the continuous disease surveillance, expanding the scale and efficiency of diagnostic studies across China’s vast healthcare network. Now a day the companies are adopting hybrid and decentralized clinical evaluation models to accelerate assay validation, multicenter trials, and post-market performance studies. The integration of advanced analytics, predictive modeling, and real-world evidence strengthens post-market surveillance, enhances compliance with NMPA IVD regulations, and accelerates the commercialization of innovative diagnostic kits, molecular assays, and point-of-care testing solutions in the Chinese market.
China In Vitro Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 5.8 Billion |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR Of 7.23% |
| 2035 Value Projection: | USD 12.5 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 123 |
| Segments covered: | By Product, By Test Location |
| Companies covered:: | Thermo Fisher Scientific Inc., Sysmex Corporation, Maccura Biotechnology, Siemens Healthcare GmbH., Abbott, bioMérieux SA, Mindray Medical International Limited, Danaher Corporation, Bio-Rad Laboratories, Inc., Shanghai Kehua Bio-Engineering Co. Ltd., and Other key players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Market Dynamics of the China In Vitro Diagnostics Market
The China in vitro diagnostics (IVD) market is driven by rising healthcare demand, increasing prevalence of chronic and infectious diseases, growing disposable incomes, and the strong government support for public health and medical innovation. There is demand for the high-quality diagnostic kits, reagents, molecular assays, and the point-of-care testing is fueling both domestic innovation and imports. Spending on early diagnosis and preventative screening is rising due to rapid urbanization, the growth of the middle class, and increased health consciousness, while hospital modernization and laboratory improvements are encouraging the use of sophisticated testing platforms. Government initiatives such as the Healthy China 2030 plan, national disease-control programs, and expanded screening mandates, along with the integration of digital health, automation, and AI-enabled diagnostics, are driving sustained growth across China’s IVD industry.
The China in vitro diagnostics (IVD) market is restrained by intense domestic competition and pricing pressure from volume-based procurement, stringent and evolving NMPA regulatory requirements, reimbursement and hospital budget constraints, dependence on imported high-end reagents and core technologies, data security and localization rules, and geopolitical uncertainties affecting supply chains, which together slow commercialization and pressure margins for both domestic and foreign IVD companies.
The future of China’s in vitro diagnostics (IVD) market appears bright and promising, driven by opportunities from digital health integration, AI-enabled diagnostics, real-world evidence generation, and hybrid or decentralized clinical studies. Advanced analytics, connected instruments, smart laboratory automation, and electronic data systems are improving test accuracy, workflow efficiency, disease surveillance, and compliance with evolving NMPA regulations. In parallel, precision medicine, companion diagnostics, population screening programs, and expanding hospital and primary-care testing capacities are accelerating innovation, post-market monitoring, and faster market access for novel IVD solutions across China’s healthcare ecosystem.
Market Segmentation
The China in vitro diagnostics market share is classified into product and test location.
By Product
The China in vitro diagnostics market is divided by product into instruments, services. Among these, the instruments segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. It is due to high testing volumes, ongoing R&D for faster disease detection.
By Test Location
The China in vitro diagnostics market is divided by test location into point of care, home care. Among these, the point of care segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. It is because the POC tests provide quick results near the patient, which facilitates faster clinical decision-making and immediate treatment in non-laboratory settings like hospitals and clinics.
Competitive Analysis
The report offers the appropriate analysis of the key organisations/companies involved within the China in vitro diagnostics market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
Top Key Companies in China In Vitro Diagnostics Market
- Thermo Fisher Scientific Inc.
- Sysmex Corporation
- Maccura Biotechnology
- Siemens Healthcare GmbH.
- Abbott
- bioMérieux SA
- Mindray Medical International Limited
- Danaher Corporation
- Bio-Rad Laboratories, Inc.
- Shanghai Kehua Bio-Engineering Co. Ltd.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the China, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the China in vitro diagnostics market based on the below-mentioned segments:
China In Vitro Diagnostics Market, By Product
- Instruments
- Services.
China In Vitro Diagnostics Market, By Animal Type
- Point of Care
- Home Care.
Frequently Asked Questions (FAQ)
-
Q: What is the China in Vitro Diagnostics market size?A: China in vitro diagnostics market is expected to grow from USD 5.8 billion in 2024 to USD 12.5 billion by 2035, growing at a CAGR of 7.23% during the forecast period 2025-2035.
-
Q: What are the key growth drivers of the market?A: Market growth is driven by rising healthcare demand, increasing prevalence of chronic and infectious diseases, growing disposable incomes, and the strong government support for public health and medical innovation.
-
Q: What factors restrain the China in Vitro Diagnostics market?A: Constraints include the intense domestic competition and pricing pressure from volume-based procurement, stringent and evolving NMPA regulatory requirements.
-
Q: How is the market segmented by product?A: The market is segmented into instruments, services.
-
Q: Who are the key players in the China in Vitro Diagnostics market?A: Key companies include Abbott, bioMérieux SA, Mindray Medical International Limited, Danaher Corporation, Bio-Rad Laboratories, Inc., Shanghai Kehua Bio-Engineering Co. Ltd., Thermo Fisher Scientific Inc., Sysmex Corporation, Maccura Biotechnology, and Siemens Healthcare GmbH and Others.
-
Q: Who are the target audiences for this market report?A: The report targets market players, investors, end-users, government authorities, consulting and research firms, venture capitalists, and value-added resellers (VARs).
Need help to buy this report?