China Cervical Cancer Diagnostic Market Size, Share, By Type (Screening Test, Imaging Test, Cervical Biopsy, and Others), By Disease Stage (Stage I, Stage II, Stage III, Stage IV), and China Cervical Cancer Diagnostic Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareChina Cervical Cancer Diagnostic Market Size Insights Forecasts to 2035
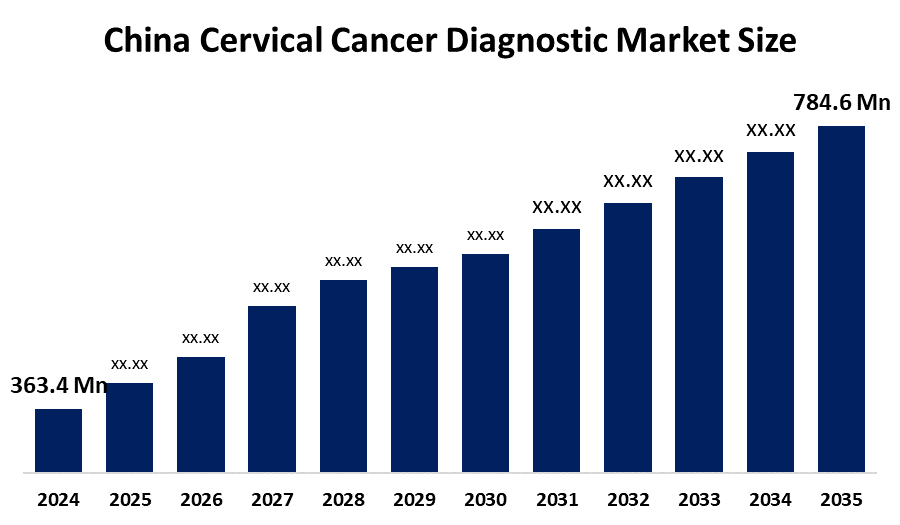
- China Cervical Cancer Diagnostic Market Size 2024: USD 363.4 Mn
- China Cervical Cancer Diagnostic Market Size 2035: USD 784.6 Mn
- China Cervical Cancer Diagnostic Market CAGR: 7.25%
- China Cervical Cancer Diagnostic Market Segments: Type and Disease Stage
Get more details on this report -
The screening, detection, and confirmatory diagnostic instruments used to detect cervical cancer and precancerous lesions in their early stages make up the China cervical cancer diagnostic market. Pap smear screenings, HPV DNA testing, liquid-based cytology, colposcopy, and biopsy procedures are important diagnostic techniques. Because early diagnosis greatly enhances treatment outcomes and survival rates, the market is vital to China's women's healthcare environment. Growing use of molecular diagnostics, growing hospital and diagnostic infrastructure, and growing awareness of preventive healthcare have all contributed to market expansion in both urban and semi-urban areas.
Through nationwide screening programs aimed at women, especially in rural and low-income areas, the Chinese government actively promotes the identification of cervical cancer. Public health initiatives under women's health and maternal care programs include cervical cancer screening. Enhancing early detection rates, funding screening services, and increasing access to healthcare through primary medical facilities are the main goals of policy initiatives. To guarantee accurate and trustworthy testing across the country, regulatory bodies also place a strong emphasis on quality control, standardization, and approval of cutting-edge diagnostic technology.
With the increasing use of liquid-based cytology, automated laboratory methods, and HPV molecular testing, technological developments are changing the industry. AI-assisted cytology image processing, digital pathology, and high-throughput testing systems are examples of emerging breakthroughs that facilitate large-scale population screening in China by improving screening efficiency, decreasing human error, and increasing diagnosis accuracy.
Market Dynamics of the China Cervical Cancer Diagnostic Market:
Growing awareness of women's health and the increasing incidence of cervical cancer are driving the Chinese cervical cancer diagnostic market. Government-run screening initiatives have greatly raised early detection rates and diagnostic volumes, particularly in underserved and rural areas. Further bolstering market expansion include increased access to healthcare facilities, better reimbursement coverage, and rising use of Pap smear and HPV testing. Additionally, routine screening and follow-up testing are being encouraged by advancements in laboratory diagnoses, increased female involvement in preventive healthcare, and increased public health education.
The market is facing a number of difficulties despite its good trend. Early diagnosis is hampered in rural and low-income locations by limited awareness and screening participation. Market expansion is further hampered by unequal access to contemporary diagnostic facilities and the high prices of sophisticated molecular diagnostic testing. Widespread adoption of advanced diagnostic methods is further hampered by a lack of qualified pathologists and cytologists as well as workflow inefficiencies in public institutions.
The development of point-of-care diagnostic technologies, liquid-based cytology, and HPV DNA testing have significant prospects. Accuracy can be increased and burden can be decreased in pathology labs by integrating AI-assisted image analysis and automation. Furthermore, telepathology, public-private collaborations, and the expansion of private diagnostic centers offer significant potential for enhancing screening coverage and propelling long-term market growth in China.
Market Segmentation
The China cervical cancer diagnostic market share is classified into type and disease stage.
By Type:
The market is categorized by type into screening tests, imaging tests, cervical biopsy, and others. Among these, the screening tests segment held the majority market share in 2024 and is predicted to grow at a remarkable rate. Increased use of Pap smear and HPV tests, government-sponsored screening programs, growing awareness of early cervical cancer detection, and the growth of regular women's health examinations throughout China.
By Disease Stage:
This market is divided by disease stage into stage I, stage II, stage III, and stage IV. Among these, the stage I segment accounted for the largest market share in 2024 and is expected to grow at a significant rate of CAGR during the projected period. China's greater focus on early diagnosis through extensive screening programs, better access to diagnostic services, growing public awareness, and improved clinical outcomes linked to early-stage cervical cancer discovery.
Competitive Analysis:
The report offers the appropriate analysis of the key organisations/companies involved within the China cervical cancer diagnostic market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
Top Key Companies in the China Cervical Cancer Diagnostic Market:
- Sansure Biotech Inc.
- Guangdong Hybribio Biotech Co., Ltd.
- Hotgen Biotech
- Shanghai Kehua Bio-Engineering Co., Ltd.
- Roche Diagnostics
- Hologic, Inc.
- Qiagen N.V.
- Abbott Laboratories
- Becton, Dickinson and Company (BD)
- Thermo Fisher Scientific
- Siemens Healthineers
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the China, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the China cervical cancer diagnostic market based on the following segments:
China Cervical Cancer Diagnostic Market, By Type
- Screening Test
- Imaging Test
- Cervical Biopsy
- Others
China Cervical Cancer Diagnostic Market, By Disease Stage
- Stage I
- Stage II
- Stage III
- Stage IV
Frequently Asked Questions (FAQ)
-
1. What is the projected China cervical cancer diagnostic market size by 2035?The market is expected to reach USD 784.6 million by 2035, growing from USD 363.4 million in 2024.
-
2. What is the expected CAGR during the forecast period?The market is projected to grow at a CAGR of 7.25% during 2025–2035.
-
3. Which type of diagnostic test holds the largest market share in China's cervical cancer diagnostic?Screening tests, including Pap smear and HPV DNA testing, dominated in 2024 due to government screening programs and growing awareness of early detection.
-
4. Which disease stage accounts for the largest market share?Stage I cervical cancer accounted for the largest share in 2024, driven by early detection initiatives and improved access to diagnostic services.
Need help to buy this report?