Global Cell and Gene Therapy Market Size, Share, and COVID-19 Impact Analysis, By Therapeutic Class (Cardiovascular Disease, Cancer, Genetic Disorder, Rare Diseases, Oncology, Hematology, Ophthalmology, Infectious Disease, Neurological Disorders, and Others), By End User (Hospitals, Cancer Care Centers, and Others), By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 - 2035
Industry: HealthcareGlobal Cell and Gene Therapy Market Insights Forecasts to 2035
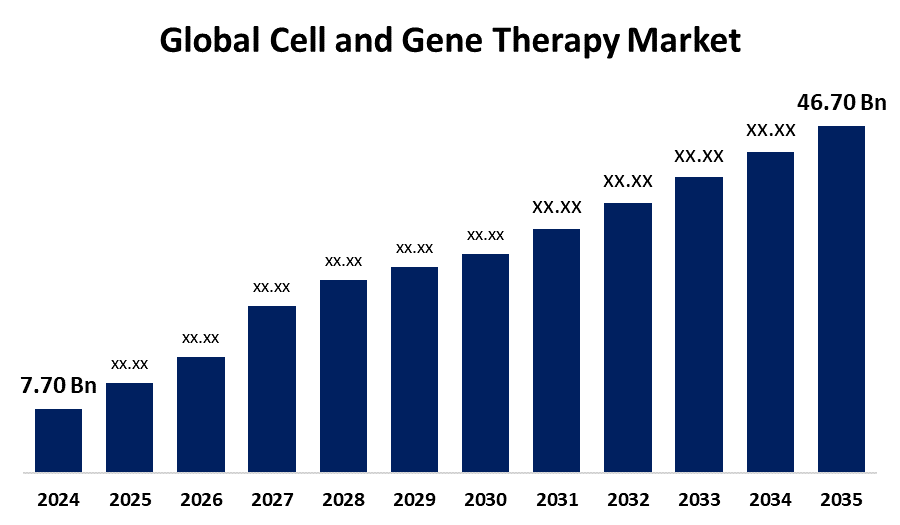
- The Global Cell and Gene Therapy Market Size Was Estimated at USD 7.70 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of around 17.81% from 2025 to 2035
- The Worldwide Cell and Gene Therapy Market Size is Expected to Reach USD 46.70 Billion by
- Asia Pacific is expected to Grow the fastest during the forecast period.
Get more details on this report -
According to a Research Report Published by Spherical Insights and Consulting, The Global Cell and Gene Therapy Market Size was worth around USD 7.70 Billion in 2024, Growing to USD 8.84 Billion in 2025, and is predicted to Grow to around USD 46.70 Billion by 2035 with a compound annual growth rate (CAGR) of 17.81% from 2025 to 2035. The market for cell and gene therapy presents game-changing opportunities due to novel chronic illness therapies, increased production capabilities, strategic alliances, substantial research funding, and regulatory developments that improve accessibility and commercialization.
Global Cell and Gene Therapy Market Forecast and Revenue Size
- 2024 Market Size: USD 7.70 Billion
- 2025 Market Size: USD 8.84 Billion
- 2035 Projected Market Size: USD 46.70 Billion
- CAGR (2025-2035): 17.81%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
Market Overview
The market for cell and gene therapy (CGT) encompasses the dynamic business environment devoted to cutting-edge biomedical methods that use genetic and cellular resources for illness diagnosis, prevention, or treatment. Cell therapy, which frequently uses autologous or allogeneic sources such as stem cells or immune cells, is the administration or transplantation of viable cells to replace, repair, or regenerate damaged tissues and organs. In contrast, gene therapy involves altering, adding to, or removing genetic sequences in a patient's cells to correct underlying genetic flaws. This process is usually accomplished using viral vectors or gene-editing tools such as CRISPR-Cas9.
The small biotech businesses creating gene and cell therapies are forced to form strategic partnerships with contract manufacturers due to a lack of funding, infrastructure, and capacity, which has stimulated sector growth. Furthermore, exponential growth in the clinical pipeline and a rise in regulatory approvals for novel medications have been the main drivers of the cell and gene therapy market. Reinforcing this momentum, the FDA launched new initiatives to advance cell and gene therapies, reflecting growing industry optimism. Regulated as biological products under CBER, these therapies require a Biologics License Application (BLA) under Section 351 before entering the U.S. market.
Key Market Insights
- North America is expected to account for the largest share in the cell and gene therapy market during the forecast period.
- In terms of therapeutic class, the infectious disease segment is projected to lead the cell and gene therapy market throughout the forecast period
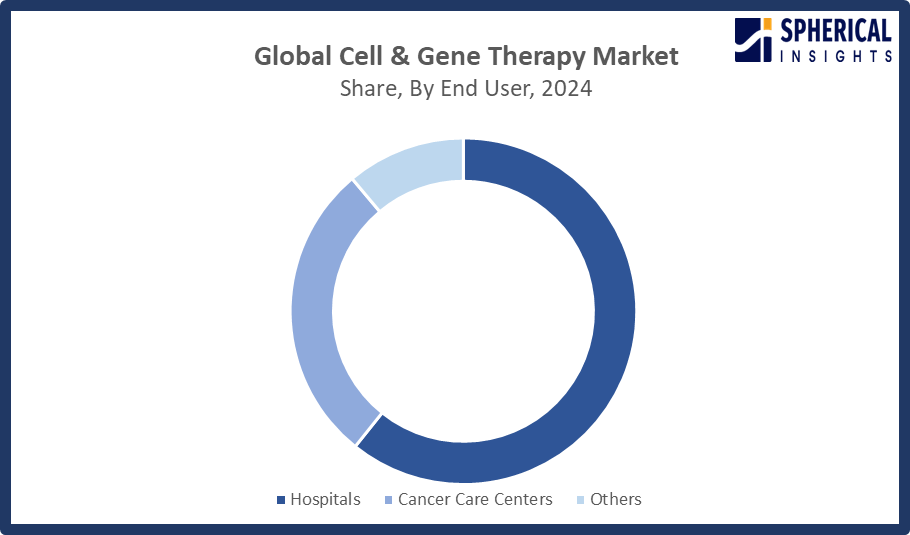
- In terms of end user, the hospital segment captured the largest portion of the market
Cell and Gene Therapy Market Growth Factors
- Accelerated Regulatory Support: To expedite the research and approval of CGTs, organizations such as the FDA and EMA are offering fast-track designations and guidelines.
- Increase in Clinical Pipelines: More and more CGT candidates, particularly those that target genetic abnormalities, uncommon diseases, and malignancies, are making their way into clinical trials.
- Manufacturing Advancements: Conventional manufacturing bottlenecks are being addressed by innovations in automated and scalable production techniques.
- Increased Commercialization Efforts: Businesses are concentrating on price plans, patient access, and long-term safety monitoring as more CGTs are approved.
Report Coverage
This research report categorizes the cell and gene therapy market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyzes the key growth drivers, opportunities, and challenges influencing the cell and gene therapy market. Recent market developments and competitive strategies, such as expansion, type launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyzes their core competencies in each sub-segment of the cell and gene therapy market.
Global Cell and Gene Therapy Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 7.70 Billion |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 17.81% |
| 2035 Value Projection: | USD 46.70 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 251 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Therapeutic, By End User and COVID-19 Impact Analysis |
| Companies covered:: | Pfizer Inc., Amgen Inc., Biogen Inc., Novartis AG, CORESTEM Inc., Helixmith Co. Ltd., Kolon TissueGene Inc., JCR Pharmaceuticals Co. Ltd., Alnylam Pharmaceuticals Inc., Dendreon Pharmaceuticals LLC., and Others |
| Pitfalls & Challenges: | COVID-19 Impact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving factors:
Rising chronic diseases drive cell gene therapy.
The growing number of people with autoimmune diseases, genetic problems, and chronic illnesses like cancer is a major factor propelling the market for cell and gene therapy. Private investors continue to invest in the cell and gene therapy market due to the potential for a single shot to give patients long-lasting clinical advantages, the exponential growth of the clinical pipeline, and the growing number of regulatory approvals for novel medications. These factors are driving the market. The revenue growth of the contract manufacturing industry has been driven by ongoing clinical research programs. The segment's growth is further supported by the growing outsourcing of the cell and gene therapy manufacturing process.
Restraining Factor:
High costs and regulations limit cell gene therapy.
The market for cell and gene therapy is restricted by factors such as high treatment costs, complicated manufacturing scalability, restricted reimbursement schemes, and regulatory issues that prevent widespread adoption, affordability, and production efficiency even in the face of scientific improvements.
Market Segmentation
The global cell and gene therapy market is divided into therapeutic class and end user.
Global Cell and Gene Therapy Market, By Therapeutic Class:
Why does the infectious disease segment hold the largest revenue share in the global cell and gene therapy market?
The infectious disease segment led the cell and gene therapy market, generating the largest revenue share. The need for cell and gene therapy for infectious disorders is growing as a result of the COVID-19 pandemic and the increase in infectious disease incidence. Antibiotic resistance is driving projections, and a growing pipeline of more than 300 studies places treatments for infectious diseases at the center of the development of precision medicine. The growing need for CRISPR/Cas and other genome editing techniques for infectious illnesses is driving the industry.
The oncology segment in the cell and gene therapy market is expected to grow at the fastest CAGR over the forecast period. The segment's growth is driven by the increasing incidence of cancer, the most recent advancements, and supportive government measures to treat cancer effectively. The oncology sector has seen the most progress in identifying and treating the many kinds of tumors as a result of these cell and gene therapies.
Global Cell and Gene Therapy Market, By End User:
What factors make hospitals suitable for delivering cell and gene therapy?
The hospitals segment held the largest market share in the cell and gene therapy market. Hospitals offer all the tools required to deliver suitable cell and gene therapies, including skilled personnel. The hospital segment can be explained by hospitals' sophisticated delivery systems, specialized medical staff, and advanced infrastructure, which enable them to offer complex therapies that need careful monitoring.
Get more details on this report -
The cancer care centers segment in the cell and gene therapy market is expected to grow at the fastest CAGR over the forecast period. The rising incidence of cancer and the growing need for individualized, cutting-edge treatment choices provided by gene and cell treatments are the main drivers of the cancer care centers market.
Regional Segment Analysis of the Global Cell and Gene Therapy Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
North America Cell and Gene Therapy Market Trends

Get more details on this report -
What factors contribute to North America region holding the largest share, approximately 49.85%, of the global cell and gene therapy market during the forecast period?
The high frequency of chronic diseases like cancer, heart disease, and genetic disorders is driving significant growth in the North American market for cell and gene therapy. The location with the most gene therapy clinical trials was North America, where over 400 businesses are actively working on developing cell and gene therapy treatments for a range of illnesses. The underlying causes of many diseases are being addressed by cell and gene therapies, which have become promising options for care and treatment.
Why does the United States lead the North American cell and gene therapy market?
The nation is a desirable market for cell and gene therapy businesses due to its huge patient population, well-established biotechnology sector, and supportive regulatory framework. The United States is home to the headquarters of several significant industry companies, which stimulate innovation and market growth.
Why is the cell and gene therapy market in Canada expanding rapidly?
The market for cell and gene therapy in Canada, a crucial area of the biopharmaceutical industry in North America, is expanding rapidly because to significant government spending in biomanufacturing and healthcare. However, Novartis Pharmaceuticals' Kymriah, the first gene therapy approved by Health Canada for the treatment of leukemia, was approved 20 years after Vitravene was licensed in the USA.
Asia Pacific Cell and Gene Therapy Market Trends
Why does the Asia Pacific region present substantial opportunities for the development and marketing of cell and gene therapy?
The Asia Pacific area presents substantial opportunities for the development and marketing of cell and gene treatments due to its diversified population, rising chronic illness prevalence, and rising healthcare infrastructure investments. A number of Asia-Pacific nations have demonstrated a significant commitment to the advancement of gene and cell therapies through government initiatives and supporting regulatory frameworks. The Asia-Pacific area is propelled by strong clinical research in rare illnesses and oncology, supported by significant government funding and regulatory changes.
Why is China expected to register the highest CAGR in the cell and gene therapy market during the forecast period?
Prominent in international clinical trials, the Chinese cell and gene therapy market focuses on rare diseases and oncology and is propelled by national initiatives. It encourages innovation and establishes China as a major biotech hub in Asia-Pacific with strong development prospects, bolstered by significant government investments and regulatory reforms. Significant investments have also been made in the field by China, which launched the Made in China 2025 project to encourage the creation of novel treatments.
What is the purpose of the Act on the Safety of Regenerative Medicine in Japan?
The Act on the Safety of Regenerative Medicine was put into effect in Japan with the intention of expediting the approval procedure for items involving regenerative medicine. Japan is making steady progress due to the Act on the Safety of Regenerative Medicine, which expedites the licensing of novel treatments for cancer, chronic illnesses, and genetic diseases.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global cell and gene therapy market, along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
Worldwide Top Key Players in The Cell and Gene Therapy Market Include
- Pfizer Inc.
- Amgen Inc.
- Biogen Inc.
- Novartis AG
- CORESTEM Inc.
- Helixmith Co. Ltd.
- Kolon TissueGene Inc.
- JCR Pharmaceuticals Co. Ltd.
- Alnylam Pharmaceuticals Inc.
- Dendreon Pharmaceuticals LLC.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent development
- In April 2025, the “Gene and Cell Therapies: The Path to Clinic” conference in Wenzhou, researchers launched cutting-edge innovations in base and prime editors, safer AAV delivery systems, and novel CAR-T/CAR-NK therapies targeting autoimmune, neurodegenerative, and sensory-organ diseases.
- In February 2025, Cell and gene therapies (CGTs) have reached 76 globally approved products since 2004, with the market forecast to exceed USD 40 billion by 2027. Key advances include CRISPR-Cas9 therapy approvals and growing use of AI, automation, and non-viral delivery systems.
- In November 2024, the American Heart Association released a science advisory highlighting advances in gene therapy for cardiovascular disease. It reviews monogenic and polygenic mechanisms, CRISPR-based editing tools, delivery challenges, and clinical trial progress, emphasizing gene therapy’s transformative potential in CVD treatment.
- In October 2022, Pfizer announced the successful acquisition of Biohaven Pharmaceuticals, a manufacturer of migraine medications. NURTEC ODT (rimegepant), which they just manufactured, is authorized for both acute treatment and the prevention of adult episodic migraines. Pfizer will be able to provide migraine sufferers additional treatment options thanks to this acquisition and their global presence.
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the cell and gene therapy market based on the following segments:
Global Cell and Gene Therapy Market, By Therapeutic Class
- Cardiovascular Disease
- Cancer
- Genetic Disorder
- Rare Diseases
- Oncology
- Hematology
- Ophthalmology
- Infectious Disease
- Neurological Disorders
- Others
Global Cell and Gene Therapy Market, By End User
- Hospitals
- Cancer Care Centers
- Others
Global Cell and Gene Therapy Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the cell and gene therapy market over the forecast period?The global cell and gene therapy market is projected to expand at a CAGR of 17.81% during the forecast period.
-
2. What is the market size of the cell and gene therapy market?The global cell and gene therapy market size is expected to grow from USD 7.70 billion in 2024 to USD 46.70 billion by 2035, at a CAGR 17.81% of during the forecast period 2025-2035.
-
3. Which region holds the largest share of the cell and gene therapy market?North America is anticipated to hold the largest share of the cell and gene therapy market over the predicted timeframe.
-
4. Who are the top companies operating in the global cell and gene therapy market?Pfizer Inc., Amgen Inc., Biogen Inc., Novartis AG, CORESTEM Inc., Helixmith Co. Ltd., Kolon TissueGene Inc., JCR Pharmaceuticals Co. Ltd., Alnylam Pharmaceuticals Inc., Dendreon Pharmaceuticals LLC, and others.
-
5. What factors are driving the growth of the cell and gene therapy market?Several factors driving the growth of the cell and gene therapy market include advancements in biotechnology, increasing prevalence of genetic disorders, rising investments, regulatory support, and growing demand for personalized medicine.
-
6. What are market trends in the cell and gene therapy market?Key trends in the cell and gene therapy market include technological advancements, strategic partnerships, increased regulatory approvals, growing clinical trials, expanding applications in rare diseases, and rising investments from pharmaceutical companies.
-
7. What are the main challenges restricting wider adoption of the cell and gene therapy market?The main challenges restricting wider adoption of cell and gene therapy include high treatment costs, complex manufacturing processes, limited infrastructure, regulatory hurdles, scalability issues, and concerns regarding long-term safety and efficacy.
Need help to buy this report?