Global CAR T-Cell Therapy Market Size, Share, and COVID-19 Impact Analysis, By Drug Type (Axicabtagene Ciloleucel, Lisocabtagene Maraleucel, Brexucabtagene Autoleucel, Idecabtagene Vicleucel, Tisagenlecleucel, Ciltacabtagene Autoleucel, Others), By Indication (Non-Hodgkin Lymphoma, Multiple Myeloma, Acute Lymphoblastic Leukemia, Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
Industry: HealthcareGlobal CAR T-Cell Therapy Market Insights Forecasts to 2033
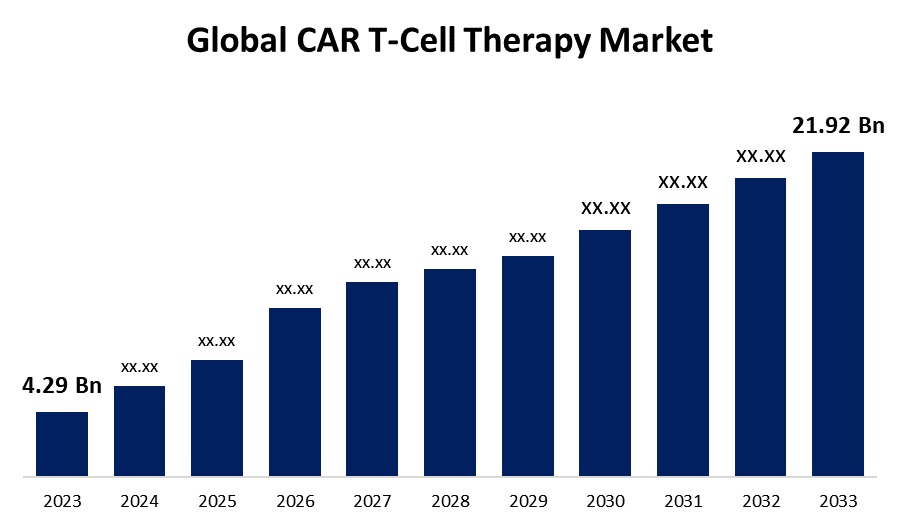
- The Global CAR T-Cell Therapy Market Size was Valued at USD 4.29 Billion in 2023
- The Market Size is Growing at a CAGR of 17.72% from 2023 to 2033
- The Worldwide CAR T-Cell Therapy Market Size is Expected to Reach USD 21.92 Billion by 2033
- Asia Pacific is expected to Grow the fastest during the forecast period.
Get more details on this report -
The Global CAR T-Cell Therapy Market Size is Anticipated to Exceed USD 21.92 Billion by 2033, Growing at a CAGR of 17.72% from 2023 to 2033.
Market Overview
The use of T cells, which are immune cells, that have undergone genetic modification in a lab to enhance their capacity to identify and eradicate cancer cells, is known as chimeric antigen receptor (CAR) T-cell therapy. In cases when other treatments are failing, CAR T-cell therapy can be incredibly successful in treating certain cancers. In addition, over the projected period, rising cancer incidence is anticipated to fuel market expansion for CAR T-cell therapy globally. For instance, the World Health Organization (WHO) released a data sheet in February 2022 stating that 2.26 million cases of breast cancer were discovered worldwide in 2021 and that approximately 400,000 children worldwide are diagnosed with cancer every year. Furthermore, CAR T-cell therapy offers a highly focused therapeutic approach that selectively targets cancerous cells while sparing healthy cells. It is anticipated that these benefits will further spur growth. Furthermore, the research stream of the firms in healthcare has been further strengthened by the discovery of various additional molecular targets resulting from the ongoing activities in the field. The market for CAR T-cell therapy is headed to become one of the most valuable segments of the biopharmaceutical sector, owing to the steady rise in the quantity of CAR T-cell therapies being created and introduced.
Report Coverage
This research report categorizes the market for the global CAR T-cell therapy market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global CAR T-cell therapy market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global CAR T-cell therapy market.
Global CAR T-Cell Therapy Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 4.29 Billion |
| Forecast Period: | 2023 - 2033 |
| Forecast Period CAGR 2023 - 2033 : | 17.72% |
| 2033 Value Projection: | USD 21.92 Billion |
| Historical Data for: | 2019 - 2022 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Drug Type, By Indication, By Region |
| Companies covered:: | Sorrento Therapeutics, Inc., Novartis AG, GSK plc., Gilead Sciences, Inc., Bristol-Myers Squibb Company, JW Therapeutics (Shanghai) Co., Ltd., Sangamo Therapeutics, bluebird bio, Inc., Johnson & Johnson Services, Inc., Merck & Co., Inc., and Others |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The production of CAR T-cells for usage in therapeutic regions is anticipated to increase in response to rising cancer incidence. It is commonly known that a variety of cancers might be treated with modified T-cell receptors. Because chimeric immunoreceptors help T-cells locate and eliminate cancer cells that possess the particular protein that the receptor can bind to, their use is on the rise. Furthermore, research and development for the use of chimeric antigen receptors in the biotechnology and life science fields is constantly expanding to treat cancer. Research on the efficacy of CAR T-cell therapy is expanding across national borders, which will facilitate the improvement of data availability about the treatment's effectiveness, mode of action, and patient compliance for leukemia and lymphoma patients. In addition, the superior efficacy of CAR T-cell therapy in comparison to other traditional medications for the treatment of cancer is anticipated to propel market expansion. In contrast to traditional medical interventions like radiation or chemotherapy, which frequently have limited specificity and systemic toxicity, CAR T-cell therapy specifically targets cancer cells, providing an extremely efficient and focused treatment.
Restraining Factors
The increased expense of medications for chimeric antigen receptor T-cell treatment and increased out-of-pocket expenses, restrict the market's expansion. The use of these treatments is constrained by their higher cost, which is a result of both their many benefits and the substantial expenses associated with their development and approval. The fact that this innovative cancer treatment is only available to people who have had extensive pretreatment presents another difficulty. Patients must have had at least two lines of systemic therapy and either relapsed or demonstrated resistance to those treatments to be eligible for tisagenlecleucel or axicabtagene ciloleucel treatment. Because of these serval factors the global CAR T-cell therapy market will be hampered during the forecasted period.
Market Segmentation
The global CAR T-cell therapy market share is classified into drug type and indication.
- The axicabtagene ciloleucel segment is expected to hold the largest share of the global CAR T-cell therapy market during the forecast period.
Based on the drug type, the global CAR T-cell therapy market is divided into axicabtagene ciloleucel, lisocabtagene maraleucel, brexucabtagene autoleucel, idecabtagene vicleucel, tisagenlecleucel, ciltacabtagene autoleucel, and others. Among these, the axicabtagene ciloleucel segment is expected to hold the largest share of the global CAR T-cell therapy market during the forecast period. This is due to the rising incidence of non-Hodgkin lymphoma, rising healthcare costs, and increased public awareness raised by various campaigns, the demand for axicabtagene ciloleucel has increased, which has led to the observed dominance.
- The non-Hodgkin lymphoma segment is expected to hold the largest share of the global CAR T-cell therapy market during the forecast period.
Based on the indication, the global CAR T-cell therapy market is divided into non-Hodgkin lymphoma, multiple myeloma, acute lymphoblastic leukemia, and others. Among these, the non-Hodgkin lymphoma segment is expected to hold the largest share of the global CAR T-cell therapy market during the forecast period. This is further exacerbated by the growing number of campaigns to enhance public awareness of these illnesses, which led to a rise in the number of diagnoses and a rise in the market for therapeutic solutions. According to the American Cancer Society, Inc.'s predicted statistics for 2023, non-Hodgkin lymphoma (NHL) will be diagnosed in roughly 80,550 people, including 44,880 men and 35,670 women, as well as adults and children.
Regional Segment Analysis of the Global CAR T-Cell Therapy Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
North America is anticipated to hold the largest share of the global CAR T-cell therapy market over the predicted timeframe.

Get more details on this report -
North America is anticipated to hold the largest share of the global CAR T-cell therapy market over the predicted timeframe. This is because hematopoietic cancer is becoming more common and is being diagnosed and treated at a higher rate. Furthermore, the region's major nations are encouraged to adopt cutting-edge medical care when sufficient compensation is provided for inpatient hospital stays. According to an article published in Avalere Health in September 2022, hospitalizations for Chimeric Antigen Receptor T-cell treatment are classified as MS-DRG 018, with a basic reimbursement amount of USD 246,958. Furthermore, technological developments in the industry and the introduction of new products by industry participants are credited with the region's success. For instance, in June 2022, the U.S. Food and Drug Administration (FDA) approved lisocabtagene maraleucel, a chimeric antigen receptor T-cell therapy targeted at CD19, for the treatment of adult cases of large B-cell lymphoma (LBCL), including diffuse large B-cell lymphoma (DLBCL). This announcement was made by the Bristol-Myers Squibb Company.
Asia Pacific is expected to grow at the fastest pace in the global CAR T-cell therapy market during the forecast period. This can be attributed to more clinical trials being conducted, patients becoming more aware of new and innovative treatments, and major industry players placing more emphasis on getting regulatory approvals before launching and distributing their products in the area. All of these factors are contributing to the growth of the market in this area. For instance, in February 2022, China's National Medical Products Administration (NMPA) approved an IND for JW Therapeutics (Shanghai) Co., Ltd. With this clearance, the company can proceed with a major clinical trial for the treatment of large B-cell lymphoma using relmacabtagene autoleucel, an autologous chimeric antigen receptor T-cell immunotherapy product that targets CD19.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global CAR T-cell therapy market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Sorrento Therapeutics, Inc.
- Novartis AG
- GSK plc.
- Gilead Sciences, Inc.
- Bristol-Myers Squibb Company
- JW Therapeutics (Shanghai) Co., Ltd.
- Sangamo Therapeutics
- bluebird bio, Inc.
- Johnson & Johnson Services, Inc.
- Merck & Co., Inc.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In March 2024, Breyanzi, a CD19-directed chimeric antigen receptor (CAR) T cell therapy, was accelerated approved by the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who have received at least two previous lines of therapy, which included a B-cell lymphoma 2 (BCL-2) inhibitor and a Bruton tyrosine kinase (BTK) inhibitor.
- In December 2022, CARsgen Therapeutics Co., Ltd. and Shanghai Cancer Institute worked together to develop a novel technology that can significantly improve T cells' anticancer properties. Developing CAR T cells that overexpressed Runx3, the research team at CARsgen and Shanghai Cancer Institute found that Run-CAR-T cells outperformed traditional chimeric antigen receptor T-cell therapy in terms of tumor reduction and durable anticancer efficacy.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the global CAR T-cell therapy market based on the below-mentioned segments:
Global CAR T-Cell Therapy Market, By Drug Type
- Axicabtagene Ciloleucel
- Lisocabtagene Maraleucel
- Brexucabtagene Autoleucel
- Idecabtagene Vicleucel
- Tisagenlecleucel
- Ciltacabtagene Autoleucel
- Others
Global CAR T-Cell Therapy Market, By Indication
- Non-Hodgkin Lymphoma
- Multiple Myeloma
- Acute Lymphoblastic Leukemia
- Others
Global CAR T-Cell Therapy Market, Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1.Which are the key companies that are currently operating within the market?Sorrento Therapeutics, Inc., Novartis AG, GSK plc., Gilead Sciences, Inc., Bristol-Myers Squibb Company, JW Therapeutics (Shanghai) Co., Ltd., Sangamo Therapeutics, bluebird bio, Inc., Johnson & Johnson Services, Inc., Merck & Co., Inc., and Others.
-
2.What is the size of the global CAR T-cell therapy market?The Global CAR T-Cell Therapy Market is expected to grow from USD 4.29 Billion in 2023 to USD 21.92 Billion by 2033, at a CAGR of 17.72% during the forecast period 2023-2033.
-
3.Which region is holding the largest share of the market?North America is anticipated to hold the largest share of the global CAR T-cell therapy market over the predicted timeframe.
Need help to buy this report?