Canada Von Willebrand Disease (VWD) Therapies Market Size, Share, and COVID-19 Impact Analysis, By Therapy Type (Plasma Therapies, Recombinant Therapies, Desmopressin, and Others), By End User (Hospitals, Specialty Clinics, and Homecare), and Canada Von Willebrand Disease (VWD) Therapies Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareCanada Von Willebrand Disease (VWD) Therapies Market Size Insights Forecasts To 2035
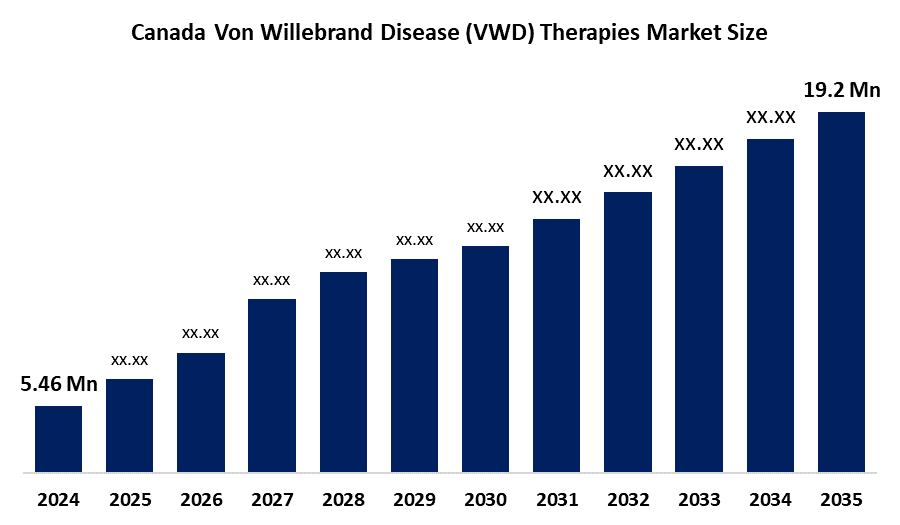
- The Canada Von Willebrand Disease (VWD) Therapies Market Size Was Estimated At USD 10.7 Million In 2024
- The Market Size Is Expected To Grow At A CAGR Of Around 5.46% From 2025 To 2035
- The Canada Von Willebrand Disease (VWD) Therapies Market Size Is Expected To Reach USD 19.2 Million By 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, The Canada Von Willebrand Disease (VWD) Therapies Market Size Is Anticipated To Reach USD 19.2 Million By 2035, Growing At A CAGR Of 5.46% From 2025 To 2035. The Canada von willebrand disease (VWD) therapies market is driven by increasing awareness of bleeding disorders, rising diagnosis rates, strong healthcare infrastructure, favourable reimbursement policies, and growing adoption of advanced plasma-derived and recombinant therapies for effective disease management.
Market Overview
The Canada von willebrand disease (VWD) therapies market size can be defined as the industry that is engaged in the development, manufacturing, and distribution of products that are used for the treatment and management of von willebrand disease, which is a hereditary bleeding disorder that is brought about by the deficiency or malfunctioning of von willebrand factor. The Canada von willebrand disease therapies market is comprised of plasma-derived von willebrand factor concentrates, recombinant von willebrand factor therapies, desmopressin, and supportive therapies.
The Canada von willebrand disease (VWD) therapies market size is experiencing major trends that are expected to influence the future growth of the market. The rising trend of adopting prophylactic treatment to prevent bleeding incidents, the increasing use of recombinant therapies owing to the enhanced safety profile, and the developments in biotechnology for the manufacture of long-acting formulations are majorly influencing the growth of the market.
The Canadian government supports the VWD therapies market size through strong public healthcare coverage, orphan drug incentives, and regulatory approvals for rare disease treatments. Health Canada’s supportive framework, combined with investments in research and development, is accelerating innovation and improving patient access to advanced therapies across the country.
Report Coverage
This research report categorizes the market size for the Canada von willebrand disease (VWD) therapies market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Canada von willebrand disease (VWD) therapies market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Canada von Willebrand disease (VWD) therapies market.
Canada Von Willebrand Disease (VWD) Therapies Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 10.7 Million |
| Forecast Period: | 2020-2023 |
| Forecast Period CAGR 2020-2023 : | 5.46% |
| 2023 Value Projection: | USD 19.2 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 250 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Therapy Type, By End User |
| Companies covered:: | Takeda Pharmaceutical Company, CSL Behring, Octa pharma, Grifols, Sanofi, and Others, Key Players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The market size for von willebrand disease (VWD) therapies in Canada is primarily driven by the rising number of diagnoses of bleeding disorders, increased awareness among patients and physicians, the rising trend of prophylactic treatment, and advancements in recombinant and plasma-derived therapies. The rising trend of long-term prophylactic treatment and home care programs is often made possible through patient support programs. There is a clear trend or preference for safer alternatives, such as recombinant von willebrand factor instead of traditional plasma device products. The strong healthcare infrastructure, favorable reimbursement environment, and steady R&D investments in rare diseases also contribute to the growth of the market.
Restraining Factors
The market size faces restraints due to the high cost of advanced VWD therapies, limited patient population owing to the rare nature of the disease, and complex manufacturing processes for plasma-derived products. Additionally, dependency on plasma supply and logistical challenges may limit market expansion.
Market Segmentation
The Canada von willebrand disease (VWD) therapies market share is classified into therapy type and end user.
- The plasma therapies segment accounted for the largest revenue market in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Canada von willebrand disease (VWD) therapies market size is segmented by therapy type into plasma therapies, recombinant therapies, desmopressin, and others. Among these, the plasma therapies segment accounted for the largest revenue market in 2024 and is expected to grow at a significant CAGR during the forecast period. Since plasma therapies have well-established clinical uses and are effective in all major types of VWD, and consequently are widely prescribed as the first-line treatment for VWD in Canada. These therapies are easily accessible in hospitals and specialty clinics, and are also well-established in clinical practices, and are preferred for the treatment of moderate to severe bleeding episodes.
- The hospitals segment accounted for the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period.
Based on end user, the Canada von willebrand disease (VWD) therapies market size is bifurcated into hospitals, specialty clinics, and home care. Among these, the hospitals segment accounted for the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period. Hospitals are at the leading edge of the market because they have specialized hematology units, and they are the main centers for the diagnosis and management of severe cases of VWD. Moreover, most plasma-derived and recombinant products are administered in a hospital facility, which further drives the revenue in the hospital-based treatment segment.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Canada von willebrand disease (VWD) therapies market size, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Takeda Pharmaceutical Company
- CSL Behring
- Octa pharma
- Grifols
- Sanofi
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the Canada, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Canada von willebrand disease (VWD) therapies market based on the below-mentioned segments:
Canada Von Willebrand Disease (VWD) Therapies Market, By Therapy Type
- Plasma Therapies
- Recombinant Therapies
- Desmopressin
- Others
Canada Von Willebrand Disease (VWD) Therapies Market, By End User
- Hospitals
- Specialty Clinics
- Homecare
Frequently Asked Questions (FAQ)
-
What is the Canada von willebrand disease (VWD) therapies market Size?Canada von willebrand disease (VWD) therapies market size is expected to grow from USD 10.7 million in 2024 to USD 19.2 million by 2035, growing at a CAGR of 5.46% during the forecast period 2025-2035.
-
What are the key drivers of the Canada von willebrand disease (VWD) therapies market?The market is driven by increasing awareness and diagnosis of bleeding disorders, advancements in plasma-derived and recombinant therapies, and strong healthcare infrastructure and reimbursement support.
-
Which therapy types dominate the Canada von willebrand disease (VWD) therapies market?Plasma von Willebrand factor therapies dominate the market due to their proven clinical efficacy, long-term usage, and wide availability across hospitals and specialty clinics.
-
What are the major trends in the Canada von willebrand disease (VWD) therapies market?Major trends include a shift toward prophylactic treatment, growing adoption of recombinant therapies, expansion of home-based care, and development of long acting formulation.
-
Who are the key companies operating in the Canada von willebrand disease (VWD) therapies market?Key companies operating in the market include Takeda Pharmaceutical Company, CSL Behring, Octapharma, Grifols, Sanofi, and Others
-
What is the future outlook for the Canada von willebrand disease (VWD) therapies market?The markets expected to grow steadily through 2025, driven by continuous therapeutic innovation, improved patient access, and supportive healthcare policies for rare diseases.
Need help to buy this report?