Global Biologics Safety Testing Market Size, Share, and COVID-19 Impact Analysis, By Test Type (Mycoplasma, Sterility, Endotoxin, Bioburden, and Virus Safety), By Application (Vaccines & Therapeutics, Blood & Blood Products, Tissue & Tissue Products, Cellular & Gene Therapy Products, and Stem Cell Products), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 - 2035.
Industry: HealthcareGlobal Biologics Safety Testing Market Insights Forecasts to 2035
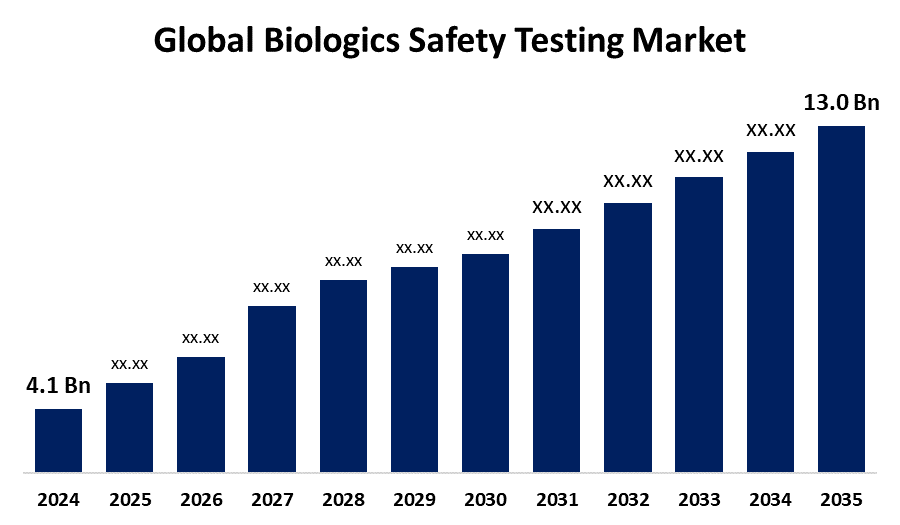
- The Global Biologics Safety Testing Market Size Was Estimated at USD 4.1 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of around 11.06% from 2025 to 2035
- The Worldwide Biologics Safety Testing Market Size is Expected to Reach USD 13.0 Billion by 2035
- Asia Pacific is expected to Grow the fastest during the forecast period.
Get more details on this report -
The Global Biologics Safety Testing Market Size was worth around USD 4.1 Billion in 2024 and is predicted to Grow to around USD 13.0 Billion by 2035 with a compound annual growth rate (CAGR) of 11.06% from 2025 to 2035. The growing focus on novel biotherapeutics and the advancement of the biopharmaceutical industry are contributing factors that drive the biologics safety testing market.
Market Overview
The biologics safety testing market refers to the industry encompassing the testing and characterization of biological products for ensuring their safety and efficacy, especially for products such as vaccines, gene therapies, and biopharmaceuticals. Biologics safety testing involves various tests for detecting and eliminating potential contaminants, confirming the product’s identity and potency, and assessing its overall safety. The services aid in verifying the absence of bacterial contaminants, thereby ensuring the safety of vaccines and biopharmaceuticals. The growing number of biopharmaceutical companies producing highly effective therapeutic drugs leads to surging emphasis on enhancing industrial processes, which is anticipated to drive the market demand for biologics safety testing. Further, the strict safety standards enforced by the regulatory authorities are driving the adoption of testing tools, which ultimately results in propelling the biologics safety testing market.
Innovative approaches and advanced technologies in the next generation therapeutics by various biotechnology and pharmaceutical organizations for improved efficacy, precision, and safety profiles are escalating market growth opportunities for biologics safety testing.
Report Coverage
This research report categorizes the biologics safety testing market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the biologics safety testing market. Recent market developments and competitive strategies such as expansion, type launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the biologics safety testing market.
Global Biologics Safety Testing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 4.1 Billion |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 11.06% |
| 2035 Value Projection: | USD 13.0 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 250 |
| Tables, Charts & Figures: | 100 |
| Segments covered: | By Test Type (Mycoplasma, Sterility, Endotoxin, Bioburden, and Virus Safety), By Application (Vaccines & Therapeutics, Blood & Blood Products, Tissue & Tissue Products, Cellular & Gene Therapy Products, and Stem Cell Products), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa) |
| Companies covered:: | Abcam, PathogenDx, Thermo Fisher Scientific, Boehringer Ingelheim, Vancouver Testing Laboratories, Sartorius AG, Wuxi AppTec, Lonza Group, Merck KGaA, SGS SA, Charles River Laboratories, Invetech, BioReliance, Eurofins Scientific, WuXi PharmaTech, and Others |
| Pitfalls & Challenges: | COVID-19 Impact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The development of monoclonal antibodies (mAbs) and biosimilars, driving the need for safety testing to ensure patient safety and product quality, is propelling the biologics safety testing market demand. Advancements in testing technology, including the adoption of automation, real-time monitoring, high-throughput screening, and integration of AI and machine learning, are driving the market growth. Government funding, as well as the need for safe and reliable testing methods in the healthcare sector, are contributing significantly to driving the market growth for biologics safety testing.
Restraining Factors
An increased cost and high capital investment required for safety testing in the development of biologics drugs are challenging the market. Animal ethical concerns and time time-consuming approval process are restricting the market expansion.
Market Segmentation
The biologics safety testing market share is classified into test type and application.
- The endotoxin segment dominated the market with a significant revenue share in 2024 and is projected to grow at a substantial CAGR during the forecast period.
Based on the test type, the biologics safety testing market is divided into mycoplasma, sterility, endotoxin, bioburden, and virus safety. Among these, the endotoxin segment dominated the market with a significant revenue share in 2024 and is projected to grow at a substantial CAGR during the forecast period. Endotoxin safety testing ensures the safety of pharmaceuticals and other medical device products that come into contact with the body, especially intravenous or injectable products. The growing application of biologics safety tests for reducing the threat of endotoxins is expected to drive the market in the endotoxin segment.
- The vaccines & therapeutics segment dominated the market with a substantial market share in 2024 and is anticipated to grow at a significant CAGR during the forecast period.
Based on the application, the biologics safety testing market is divided into vaccines & therapeutics, blood & blood products, tissue & tissue products, cellular & gene therapy products, and stem cell products. Among these, the vaccines & therapeutics segment dominated the market with a substantial market share in 2024 and is anticipated to grow at a significant CAGR during the forecast period. Biologics safety testing ensures product quality, patient safety, and efficacy in vaccines and therapeutics. The strict regulations emphasize the safety and efficacy of vaccines, prioritizing therapeutic integrity is propelling the market demand in the vaccine and therapeutics segment.
Regional Segment Analysis of the Biologics Safety Testing Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
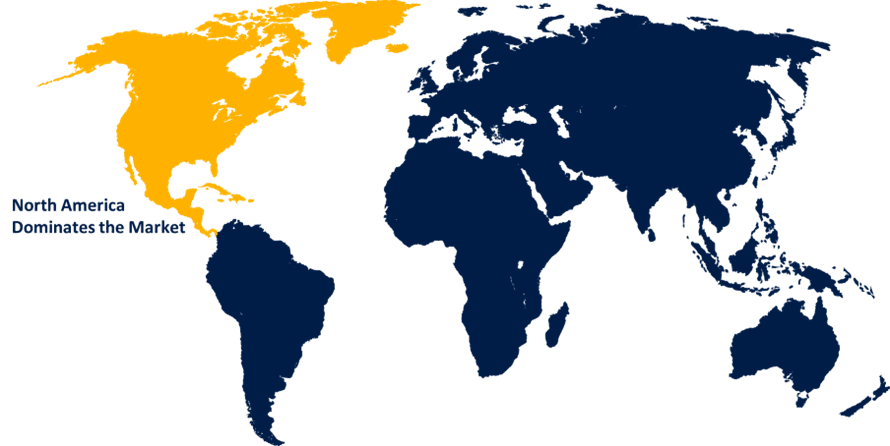
North America is anticipated to hold the largest share of the biologics safety testing market over the predicted timeframe.
Get more details on this report -
North America is anticipated to hold the largest share of the biologics safety testing market over the predicted timeframe. The demand for extensive safety assessments due to the enforcement of stringent safety standards by the USFDA and other regulatory bodies is driving the market demand. Further, the growing demand for biologics products, including mAb, vaccines, and cell treatments, due to the prevalence of chronic diseases, is driving the need for rigorous safety testing, thereby propelling the market demand.
Asia Pacific is expected to grow at a rapid CAGR in the biologics safety testing market during the forecast period. The growing investment in biopharmaceuticals with the growing development of monoclonal antibodies and biosimilars, is contributing to the biologics safety testing market. Further, the increasing need for biologics and biosimilars, along with increasing R&D activities for advancing the biotechnology industry, are propelling the market for biologics safety testing.
Europe is anticipated to hold a substantial market share of the biologics safety testing market during the predicted timeframe. An increasing demand for safe and effective biologics, along with the expanding biopharmaceutical industry, is driving the biologics safety testing market demand in the region. Further, significant R&D expenditure and the presence of well-established healthcare infrastructure and advanced therapies are contributing to propel the market growth.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the biologics safety testing market, along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Abcam
- PathogenDx
- Thermo Fisher Scientific
- Boehringer Ingelheim
- Vancouver Testing Laboratories
- Sartorius AG
- Wuxi AppTec
- Lonza Group
- Merck KGaA
- SGS SA
- Charles River Laboratories
- Invetech
- BioReliance
- Eurofins Scientific
- WuXi PharmaTech
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In January 2024, Charles River Laboratories International has advanced its flagship Endosafe cartridge technology and combined it with its recombinant cascade reagent (rCR) to launch the Endosafe Trillium rCR cartridge offering.
- In November 2023, Merck, a leading science and technology company, has completed the second phase of its new € 29 million Biologics Testing Center in China adding 1,500 square meters to the lab. These are the first biosafety laboratories for Merck in this market, enabling clients to locally access a broad range of testing services for cell line characterization and lot release from pre-clinical development to commercialization.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the biologics safety testing market based on the below-mentioned segments:
Global Biologics Safety Testing Market, By Test Type
- Mycoplasma
- Sterility
- Endotoxin
- Bioburden
- Virus Safety
Global Biologics Safety Testing Market, By Application
- Vaccines & Therapeutics
- Blood & Blood Products
- Tissue & Tissue Products
- Cellular & Gene Therapy Products
- Stem Cell Products
Global Biologics Safety Testing Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the biologics safety testing market over the forecast period?The global biologics safety testing market is projected to expand at a CAGR of 11.06% during the forecast period.
-
2. What is the market size of the biologics safety testing market?The global biologics safety testing market size is expected to grow from USD 4.1 Billion in 2024 to USD 13.0 Billion by 2035, at a CAGR of 11.06% during the forecast period 2025-2035.
-
3. Which region holds the largest share of the biologics safety testing market?North America is anticipated to hold the largest share of the biologics safety testing market over the predicted timeframe.
Need help to buy this report?