Asia Pacific Noninvasive Prenatal Testing Market Size, Share, and COVID-19 Impact Analysis, By Instruments (Ultrasound, NGS), By Method (FCMB, Cf-DNA), and Asia Pacific Noninvasive Prenatal Testing Market Insights, Industry Trends, Forecast to 2035.
Industry: HealthcareAsia Pacific Noninvasive Prenatal Testing Market Insights Forecasts to 2035
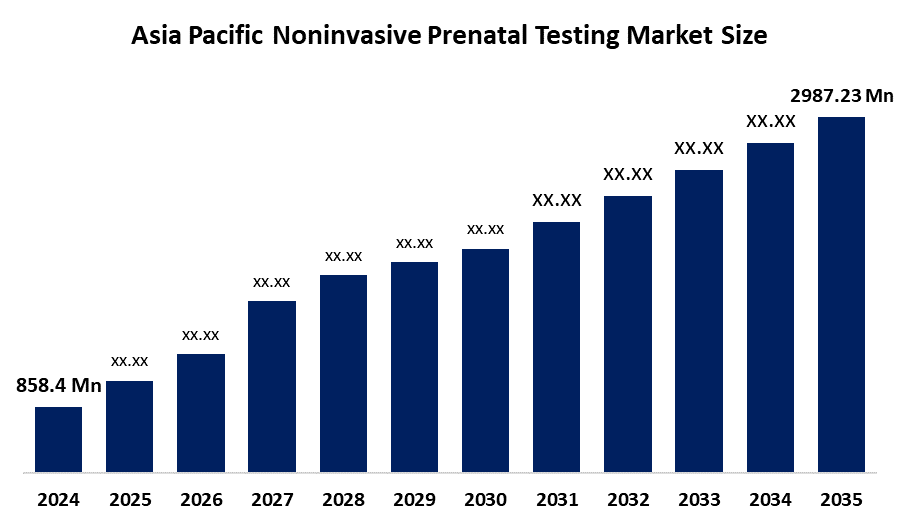
- The Asia Pacific Noninvasive Prenatal Testing Market Size Was Estimated at USD 858.4 Million in 2024
- The Market Size is Expected to Grow at a CAGR of Around 12% from 2025 to 2035
- The Asia Pacific Noninvasive Prenatal Testing Market Size is Expected to Reach USD 2987.23 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the Asia Pacific Noninvasive Prenatal Testing Market Size Is Anticipated To Reach USD 2987.23 Million By 2035, Growing At A CAGR Of 12% From 2025 To 2035. The market is driven by rising maternal age, increased awareness, supportive government policies, and technological advancements like Next-Generation Sequencing (NGS).
Market Overview
Noninvasive prenatal testing (NIPT) refers to a sophisticated screening method that analyzes fragments of fetal cell-free DNA (cfDNA) circulating in a pregnant woman's blood to assess the risk of certain genetic abnormalities, such as down syndrome. Unlike traditional invasive procedures like amniocentesis, NIPT is safe, highly accurate, and can be performed as early as the 10th week of pregnancy without risking miscarriage. Key characteristics include its reliance on Next-Generation Sequencing (NGS) and its increasing adoption as a first-line screening tool due to its minimal procedural risks.
Government and private initiatives are significantly bolstering the NIPT landscape in Asia Pacific. Nations like Singapore, Japan, and Australia have integrated NIPT into national screening programs or provided significant insurance coverage to improve affordability and access. Private healthcare institutions are also expanding their portfolios and establishing advanced diagnostic centers to meet the growing demand for personalized prenatal care.
Technological advancements, particularly in Next-Generation Sequencing (NGS) and bioinformatics, have revolutionized NIPT by enhancing its precision, speed, and scope. Modern platforms can now detect a broader range of conditions, including microdeletions and single-gene disorders, while automation in sequencing workflows has significantly reduced turnaround times.
Report Coverage
This research report categorizes the market for the Asia Pacific Noninvasive Prenatal Testing market Size based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Asia Pacific Noninvasive Prenatal Testing Market Size. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Asia Pacific noninvasive prenatal testing market.
Asia Pacific Noninvasive Prenatal Testing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 858.4 Million |
| Forecast Period: | 2024-2035 |
| Forecast Period CAGR 2024-2035 : | CAGR Of 12% |
| 2035 Value Projection: | USD 2987.23 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 210 |
| Tables, Charts & Figures: | 95 |
| Segments covered: | By Method, By By Instruments |
| Companies covered:: | BGI Genomics Co., Ltd. Berry Genomics Co. Ltd. Sonic Healthcare Limited Cordlife Group Limited Annoroad Gene Technology Macrogen Japan LSI Medience Corporation SRL, Inc. MedGenome Premas Life Sciences (India) And Others Key players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The market is primarily driven by an increasing maternal age across the Asia Pacific region, which correlates with a higher risk of chromosomal abnormalities in newborns. Additionally, the growing awareness regarding prenatal care and the rising demand for safer, non-invasive alternatives to traditional diagnostic methods are fueling adoption. Rapid healthcare infrastructure development and the expansion of insurance coverage for genetic testing in countries like China and India further accelerate market growth by making these advanced tests more accessible to the general population.
Restraining Factors
The primary restraint is the high out-of-pocket cost associated with NIPT, which remains unaffordable for many families in developing APAC nations. Furthermore, complex regulatory frameworks and a lack of skilled laboratory personnel hinder widespread implementation in rural areas.
Market Segmentation
The Asia Pacific noninvasive prenatal testing market share is categorised into Instruments and method.
- The NGS segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Asia Pacific Noninvasive Prenatal Testing Market Size is segmented by instruments into Ultrasound and NGS. Among these, the NGS segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. In the Asia-Pacific non-invasive prenatal testing (NIPT) market, next-generation sequencing (NGS) technology dominates over ultrasound due to its superior accuracy in detecting genetic conditions like Down syndrome by analyzing cell-free fetal DNA (cfDNA), offering higher sensitivity and specificity, enabling earlier, non-invasive detection of trisomies and microdeletions, and improving patient outcomes, despite ultrasound's complementary role.
- The Cf-DNA segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period.
Based on method, the Asia Pacific Noninvasive Prenatal Testing Market Size is segmented into FCMB and Cf-DNA. Among these, the Cf-DNA segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period. Cell-Free DNA (cfDNA) testing dominates over Fetal Cell-Free Maternal Blood (FCMB) because of cfDNA's superior accuracy, early detection, minimal risk (just a blood draw), and broader availability through Next-Generation Sequencing (NGS) and other advanced technologies, making it the preferred, safer alternative to invasive tests for conditions like Down Syndrome.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Asia Pacific Noninvasive Prenatal Testing Market Size, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and Noninvasive Prenatal Testing. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- BGI Genomics Co., Ltd.
- Berry Genomics Co. Ltd.
- Sonic Healthcare Limited
- Cordlife Group Limited
- Annoroad Gene Technology
- Macrogen Japan
- LSI Medience Corporation
- SRL, Inc.
- MedGenome
- Premas Life Sciences (India)
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the Asia Pacific, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Asia Pacific Noninvasive Prenatal Testing Market Size based on the below-mentioned segments
Asia Pacific Noninvasive Prenatal Testing Market, By Instruments
- Ultrasound
- NGS
Asia Pacific Noninvasive Prenatal Testing Market, By Method
- FCMB
- Cf-DNA
Frequently Asked Questions (FAQ)
-
What is the Asia Pacific noninvasive prenatal testing market size?The Asia Pacific noninvasive prenatal testing market size is expected to grow from USD 858.4 Million in 2024 to USD 2987.23 Million by 2035, growing at a CAGR of 12% during the forecast period 2025-2035
-
What are the key growth drivers of the market?The Asia Pacific noninvasive prenatal testing market is primarily driven by the rapid expansion of the electronics and automotive industries, particularly in China, Japan, and South Korea. As the global hub for semiconductor manufacturing, this region sees a constant demand for high-purity Noninvasive Prenatal Testings used in chemical vapor deposition
-
What factors restrain the Asia Pacific noninvasive prenatal testing market?Market growth is hindered by the high volatility of raw material prices and the complex environmental regulations governing chemical manufacturing. Additionally, the specialized equipment and high energy consumption required for Noninvasive Prenatal Testing synthesis can lead to significant operational costs
-
How is the market segmented by method?The market is segmented into FCMB, Cf-DNA.
Need help to buy this report?