Global Advanced Therapy Medicinal Products Market Size, Share, and COVID-19 Impact Analysis, By Therapy Type (CAR-T Therapy, Cell Therapy, Tissue Engineered Product, Gene Therapy, and Others), By Product Type (Tissue Engineered Products, Somatic Cell Treatment Product, Joined ATMPs, and Others), By Applications (Muscular Dystrophies, Alzheimer’s, Hemophilia, Cystic Fibrosis, and Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 - 2035
Industry: HealthcareGlobal Advanced Therapy Medicinal Products Market Insights Forecasts to 2035
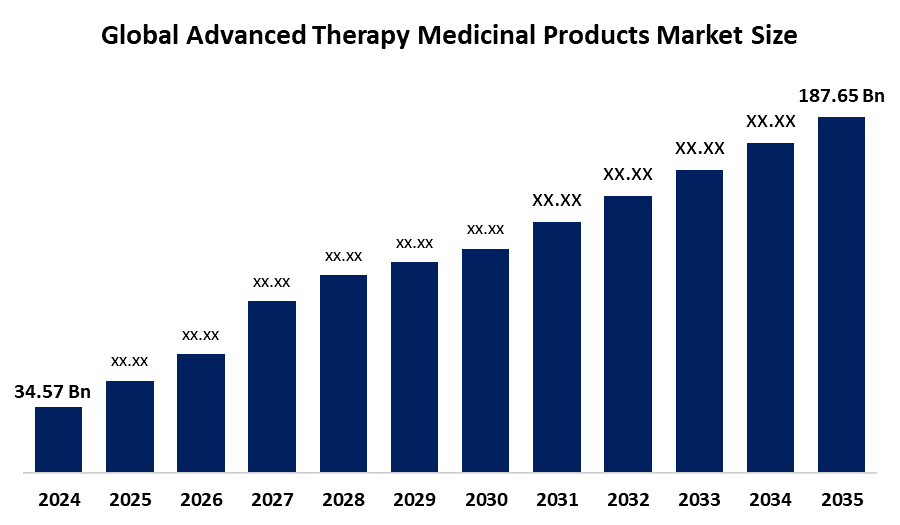
- The Global Advanced Therapy Medicinal Products Market Size Was Estimated at USD 34.57 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of around 16.62% from 2025 to 2035
- The Worldwide Advanced Therapy Medicinal Products Market Size is Expected to Reach USD 187.65 Billion by 2035
- Asia Pacific is expected to grow the fastest during the forecast period.
Get more details on this report -
According to a research report published by Spherical Insights and Consulting, The Global Advanced Therapy Medicinal Products Market size was worth around USD 34.57 Billion in 2024 and is predicted to grow to around USD 187.65 Billion by 2035 with a compound annual growth rate (CAGR) of 16.62% from 2025 to 2035. The growth in the market for advanced therapy medicinal products is attributed to increasing demand for personalized and regenerative treatments, progress in gene and cell therapy technologies, rising R&D investments, supportive regulatory frameworks, and success in managing rare and chronic diseases.
Market Overview
The Global Advanced Therapy Medicinal Products (ATMP) Market Size refers to the gene therapies, somatic cell therapies, and tissue-engineered products that are designed to repair, replace, or regenerate human cells, tissues, or organs. These are innovative therapies with enormous potential for treating life-threatening genetic disorders, cancer, and degenerative diseases. This market is mainly influenced by increasing demand for personalized medicine, genetic and chronic diseases on the rise, and rapid progress in biotechnology and regenerative medicine. Continuous innovation with gene editing tools such as CRISPR, improved viral vector production, and scalable cell culture technologies accelerates product development and commercialization. Supportive government policies, fast-track approvals by agencies including the FDA and EMA, and investments by pharmaceutical and biotechnology companies encourage growth. Artificial intelligence and automation in manufacturing processes are also furthering efficiency and reducing production costs.
Key market opportunities include the growth of therapeutic applications, increased healthcare spending, and the implementation of advanced therapies in emerging markets. The leading companies that push forward innovative ideas and partnerships within the market include Novartis AG, Gilead Sciences, Bristol Myers Squibb, Spark Therapeutics, Bluebird Bio, and Orchard Therapeutics. Continuous partnerships between research institutions and biotech firms continue to spur breakthroughs, positioning ATMPs as one of the most promising and dynamic segments of the global biopharmaceutical industry, with overwhelming potential for modern-day health management. In May 2025, the European Medicines Agency published a Concept Paper on revising the Good Manufacturing Practice (GMP) guidelines for Advanced Therapy Medicinal Products (ATMPs). The revision updates Part IV of the EU GMP Guidelines to align with the revised Annex 1 and to refine the definitions of human origin materials and include the concept of emerging technologies such as closed systems, automation, and advanced analytics.
Report Coverage
This research report categorizes the advanced therapy medicinal products market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the advanced therapy medicinal products market. Recent market developments and competitive strategies such as expansion, type launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the advanced therapy medicinal products market.
Global Advanced Therapy Medicinal Products Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 34.57 Billion |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR of 16.62% |
| 2035 Value Projection: | USD 187.65 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 244 |
| Tables, Charts & Figures: | 100 |
| Segments covered: | By Therapy Type, By Product Type |
| Companies covered:: | Novartis AG, Pfizer Inc., Spark Therapeutics, Inc., Gilead Sciences, Inc., Bristol Myers Squibb Company, Vericel Corporation, Bluebird Bio, Inc., F. Hoffmann La Roche Ltd., uniQure N.V., JCR Pharmaceuticals Co., Ltd., BioMarin, Kolon TissueGene, Inc., CRISPR Therapeutics, And Others Players |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The global advanced therapy medicinal products market is driven by the rising prevalence of chronic and genetic disorders, increasing demand for personalized medicine, and rapid advancements in gene editing and cell therapy technologies. The growing investments from pharmaceutical companies and supportive government regulations for innovative therapies also boost market growth. The expanding applications of ATMPs in oncology, rare diseases, and regenerative medicine further accelerate their adoption. Additionally, increasing success rates of clinical trials and collaborations between biotech firms and research institutions enhance product development, improving accessibility and fueling global market expansion for advanced therapeutic solutions.
Restraining Factors
The factors that will restrain the growth of the global advanced therapy medicinal products market include high development and manufacturing costs, complex regulatory requirements, and challenges associated with large-scale production. Limited reimbursement frameworks, logistical issues in handling cell and gene therapies, and concerns over safety and ethics hinder the wide adoption and commercialization of these therapies, slowing overall market growth.
Market Segmentation
The advanced therapy medicinal products market share is classified into therapy type, product type, and applications.
- The gene therapy segment dominated the market in 2024, approximately 52% and is projected to grow at a substantial CAGR during the forecast period.
Based on the therapy type, the advanced therapy medicinal products market is divided into CAR-T therapy, cell therapy, tissue engineered product, gene therapy, and others. Among these, the gene therapy segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period. the gene therapy segment dominated the advanced therapy medicinal products market owing to increasing prevalence of genetic and rare diseases, increasing demand for personalized and curative treatments, and rapid technological advancement in gene editing and delivery systems. Besides this, supportive regulatory frameworks, growing R&D investments, and successful clinical trials are driving adoption, hence making gene therapy a key growth driver in the global ATMP market.
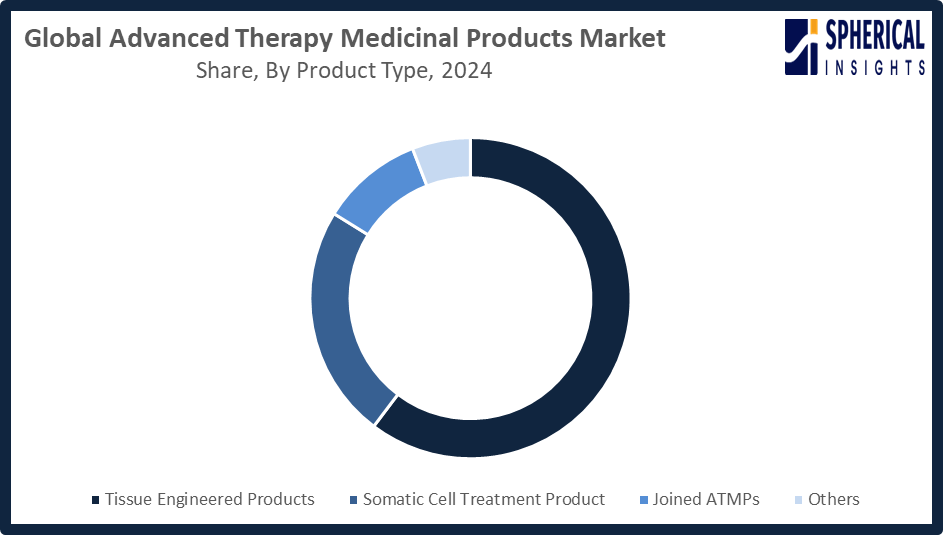
- The tissue engineered products segment accounted for the largest share in 2024, approximately 60% and is anticipated to grow at a significant CAGR during the forecast period.
Based on the product type, the advanced therapy medicinal products market is divided into tissue engineered products, somatic cell treatment product, joined ATMPs, and others. Among these, the tissue engineered products segment accounted for the largest share in 2024 and is anticipated to grow at a significant CAGR during the forecast period. The growth in the tissue-engineered products segment is attributed to increasing demand for regenerative medicine, rising prevalence of chronic and degenerative diseases, advancements in biomaterials and scaffold technologies, supportive government initiatives, and growing investments from biopharmaceutical companies developing innovative tissue-engineered therapies around the world.
Get more details on this report -
- The muscular dystrophies segment accounted for the highest market revenue in 2024, approximately 30% and is anticipated to grow at a significant CAGR during the forecast period.
Based on the applications, the advanced therapy medicinal products market is divided into muscular dystrophies, alzheimer’s, hemophilia, cystic fibrosis, and others. Among these, the muscular dystrophies segment accounted for the highest market revenue in 2024 and is anticipated to grow at a significant CAGR during the forecast period. This is attributed to the rising prevalence of muscular dystrophy globally, increasing awareness and early diagnosis, alongside great development in the area of gene and cell therapies, enabling regulatory frameworks, and rapidly growing investments from biopharmaceutical firms in developing targeted therapies for improving outcomes and the quality of life of afflicted patients.
Regional Segment Analysis of the Advanced Therapy Medicinal Products Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
North America is anticipated to hold the largest share of the advanced therapy medicinal products market over the predicted timeframe.
North America is anticipated to hold the largest share of the advanced therapy medicinal products market over the predicted timeframe. North America is expected to approximate a 48% share in the advanced therapy medicinal products market during the forecast period, driven by the United States and Canada. It owes this position to its established healthcare infrastructure, high adoption of innovative therapies, and significant R&D investments from leading biopharmaceuticals. Favorable regulatory regimes from the FDA and Health Canada, growing genetic and chronic diseases, and increasing awareness about personalized medicine also act as growth factors. Further, continuous progress in the areas of gene and cell therapies, intense clinical trial activity, and various government initiatives quicken the pace of ATMP development and market growth.
On 15 January 2024, Canada’s Access Consortium, consisting of health regulators from multiple jurisdictions, established an ATMP working group to enhance regulatory harmonization, provide shared scientific guidance, and streamline the international assessment and approval processes for advanced therapy medicinal products (ATMPs).
Asia Pacific is expected to grow at a rapid CAGR in the advanced therapy medicinal products market during the forecast period. The Asia Pacific region is experiencing rapid growth in the advanced therapy medicinal products market at a CAGR during the forecast period, with an approximate 23% market share, driven by rising healthcare investments, an increase in genetic and chronic diseases, and better awareness about advanced therapies. The growth will be majorly driven by countries such as China, Japan, and India due to favorable government initiatives, expanding infrastructure in biotechnology, and regulatory reforms that accommodate easy clinical trials and approvals. Besides this, a large pool of patient populations, improving reimbursement policies, and increased collaborations between local and global firms in biotech are driving the adoption of ATMPs within the region.
Growth in the European market for advanced therapy medicinal products is very rapid, informed by immense regulatory support via the European Medicines Agency and the ATMP Regulation (EC) No 1394/2007, which streamlines approval and ensures safety. On a country level, Germany leads due to its advanced pharmaceutical infrastructure and robust R&D investments. The UK fosters innovation by means of government funding, incentives, and accelerated regulatory pathways. In addition, increasing awareness of personalized medicine and the increasing prevalence of genetic and chronic diseases further contribute to the expanding market in Europe.
In May 2025, the European Health and Digital Executive Agency (HaDEA), under the EU’s Horizon Europe programme, launched the Optimising the Manufacturing of ATMPs call (HORIZON HLTH 2025 01 IND 01) to enhance processes, integrate AI and analytics, support flexible manufacturing, and accelerate patient access to ATMPs.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the advanced therapy medicinal products market, along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Novartis AG
- Pfizer Inc.
- Spark Therapeutics, Inc.
- Gilead Sciences, Inc.
- Bristol Myers Squibb Company
- Vericel Corporation
- Bluebird Bio, Inc.
- F. Hoffmann La Roche Ltd.
- uniQure N.V.
- JCR Pharmaceuticals Co., Ltd.
- BioMarin
- Kolon TissueGene, Inc.
- CRISPR Therapeutics
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In October 2025, JCR Pharmaceuticals announced an exclusive licensing agreement with Menagen Pharmaceutical Industries to seek marketing authorizations and commercialize Agalsidase Beta BS I.V. Infusion across nine MENAT markets, advancing access to therapies for rare and genetic diseases in the region.
- In July 2025, Bristol Myers Squibb and Bain Capital announced the creation of a new independent biopharmaceutical company focused on developing innovative therapies for autoimmune diseases, aiming to address significant unmet patient needs.
- In June 2025, BioMarin Pharmaceutical announced that an individual in Germany with severe hemophilia A received ROCTAVIAN (valoctocogene roxaparvovec-rvox), marking the first commercial administration of this gene therapy in Europe and representing a milestone in expanding access to innovative genetic treatments.
- In June 2025, Kolon TissueGene, specializing in advanced cell and gene therapies for degenerative joint and spinal diseases, highlighted its flagship product, TG-C, a novel allogeneic cell therapy currently in two pivotal Phase 3 U.S. trials involving over 1,000 knee osteoarthritis patients.
- In November 2024, Vyriad, Inc., a clinical-stage biotech focused on next-generation targeted genetic therapies, announced a strategic collaboration with Novartis to discover and develop in vivo chimeric antigen receptor (CAR) T-cell therapies, advancing innovative treatments in cellular immunotherapy.
- In August 2023, BioMarin Pharmaceutical announced that an individual in Germany with severe hemophilia A was treated with ROCTAVIAN (valoctocogene roxaparvovec-rvox), marking the first commercial use of this gene therapy in Europe and representing a significant milestone in expanding access to innovative genetic treatments.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the advanced therapy medicinal products market based on the below-mentioned segments:
Global Advanced Therapy Medicinal Products Market, By Therapy Type
- CAR-T Therapy
- Cell Therapy
- Tissue Engineered Product
- Gene Therapy
- Others
Global Advanced Therapy Medicinal Products Market, By Product Type
- Tissue Engineered Products
- Somatic Cell Treatment Product
- Joined ATMPs
- Others
Global Advanced Therapy Medicinal Products Market, By Applications
- Muscular Dystrophies
- Alzheimer’s
- Hemophilia
- Cystic Fibrosis
- Others
Global Advanced Therapy Medicinal Products Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the advanced therapy medicinal products market over the forecast period?The global advanced therapy medicinal products market is projected to expand at a CAGR of 16.62% during the forecast period.
-
2. What is the advanced therapy medicinal products market?The advanced therapy medicinal products (ATMPs) market is a rapidly growing sector for innovative biological drugs based on genes, cells, or tissues, aimed at treating diseases at their root cause rather than just symptoms.
-
3. What is the market size of the advanced therapy medicinal products market?The global advanced therapy medicinal products market size is expected to grow from USD 34.57 billion in 2024 to USD 187.65 billion by 2035, at a CAGR of 16.62% during the forecast period 2025-2035.
-
4. Which region holds the largest share of the advanced therapy medicinal products market?North America is anticipated to hold the largest share of the advanced therapy medicinal products market over the predicted timeframe.
-
5. Who are the top 10 companies operating in the global advanced therapy medicinal products market?Novartis AG, Pfizer Inc., Spark Therapeutics, Inc., Gilead Sciences, Inc., Bristol Myers Squibb Company, Vericel Corporation, Bluebird Bio, Inc., F. Hoffmann La Roche Ltd., uniQure N.V., JCR Pharmaceuticals Co., Ltd., BioMarin, Kolon TissueGene, Inc., CRISPR Therapeutics, and Others.
-
6. What factors are driving the growth of the advanced therapy medicinal products market?The growth of the advanced therapy medicinal products market is driven by technological advancements in gene and cell therapy, an increasing prevalence of rare and chronic diseases, favorable regulatory support and increasing approvals, a rise in personalized medicine demand, and strategic collaborations and investments by industry players.
-
7. What are the market trends in the advanced therapy medicinal products market?Key trends in the advanced therapy medicinal products market include the increasing focus on gene and cell therapies, significant investment in R&D for genetic and rare diseases, and the growing demand for contract development and manufacturing organizations (CDMOs).
-
8. What are the main challenges restricting the wider adoption of the advanced therapy medicinal products market?The main challenges restricting wider adoption of the advanced therapy medicinal products market include high costs and difficult reimbursement, complex manufacturing and logistics, and multifaceted regulatory hurdles.
Need help to buy this report?