Global Adeno Associated Virus Vector Manufacturing Market Size, Share, and COVID-19 Impact Analysis, By Scale of Operations (Clinical, Preclinical, and Commercial), By Therapeutic Area (Neurological Disorders, Metabolic Disorders, Ophthalmic Disorders, Muscular/Neuromuscular Disorders, and Others), By Application (Gene Therapy, Vaccine, Cell Therapy, and Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 - 2035
Industry: HealthcareGlobal Adeno Associated Virus Vector Manufacturing Market Size Insights Forecasts to 2035
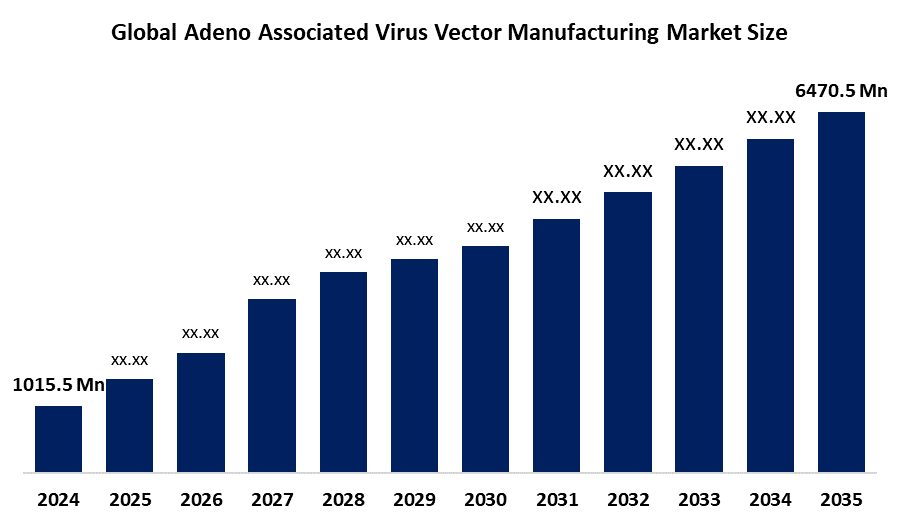
- The Global Adeno Associated Virus Vector Manufacturing Market Size Was Estimated at USD 1015.5 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 18.34% from 2025 to 2035
- The Worldwide Adeno Associated Virus Vector Manufacturing Market Size is Expected to Reach USD 6470.5 Million by 2035
- Asia Pacific is expected to Grow the fastest during the forecast period.
Get more details on this report -
According to a Research Report Published by Spherical Insights and Consulting, The Global Adeno Associated Virus Vector Manufacturing Market Size was worth around USD 1015.5 Million in 2024 and is predicted to Grow to around USD 6470.5 Million by 2035 with a compound annual growth rate (CAGR) of 18.34% from 2025 and 2035. The market for adeno-associated virus vector manufacturing has a number of opportunities to grow due to the emergence of recombinant AAV-based gene therapy for treating various diseases, namely congenital blindness, hemophilia, and spinal muscular atrophy, and other related diseases, as well as advancements in the manufacturing of adeno associated virus vector, like the integration of artificial intelligence and machine learning.
Market Overview
The global industry of adeno associated virus vector manufacturing includes the production and purification of AAV vectors, which are used for delivering therapeutic genes for treating genetic disorders, neurological diseases, and other conditions in gene therapies and vaccines. Adeno associated virus (AAV) is a non-enveloped virus that can be engineered to deliver DNA to target cells. It is considered one of the safest strategies for gene therapies due to its minimal pathogenicity and ability to establish long term gene expression in different tissues.
Innovation and market expansion are anticipated as a result of major players' growing emphasis on development of innovative cell & gene therapy and the expanding partnerships. For instance, in September 2025, Matica Biotechnology, Inc., a leading CDMO specializing in viral vector production, announced a strategic partnership with Cirsium Biosciences, a biotechnology firm pioneering plant-based AAV technology, for revolutionizing gene therapy accessibility through innovative plant-based technology and expert manufacturing support. The ongoing technological advancement in AAV manufacturing for improving efficiency, specificity, and purity of vector production is anticipated driving a huge surge in the global adeno associated virus vector manufacturing market. For instance, in February 2025, NanoMosaic published a scientific white paper detailing a study conducted with Bristol Myers Squibb (BMS) that explores the potential of nanoneedle technology as a tool for advancing the understanding of adeno-associated virus (AAV) manufacturing processes and product quality.
Report Coverage
This research report categorizes the adeno associated virus vector manufacturing market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the adeno associated virus vector manufacturing market. Recent market developments and competitive strategies such as expansion, type launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the adeno associated virus vector manufacturing market.
Global Adeno Associated Virus Vector Manufacturing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 1015.5 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 18.34% |
| 2035 Value Projection: | USD 6470.5 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 281 |
| Tables, Charts & Figures: | 135 |
| Segments covered: | By Scale of Operations, By Therapeutic Area and COVID-19 Impact Analysis |
| Companies covered:: | Oxford Biomedica PLC, GenScript (ProBio), Yposkesi, Inc., WuXi AppTec, Sarepta Therapeutics, Inc., Pfizer Inc., Roche, Audentes Therapeutics, BioMarin Pharmaceutical, F. Hoffmann-La Roche Ltd, Charles River Laboratories, Genezen, Creative Biogene, and Others |
| Pitfalls & Challenges: | COVID-19 Impact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The market demand for adeno associated virus vector manufacturing is primarily driven by the increasing need for effective treatment due to the growing prevalence of genetic disorders. For instance, the prevalence of genetic disorders in the US population is about 10% according to statistics, and only 5% of these individuals are benefiting from the treatments. Further, 30 million patients are registered with rare diseases in the European Union according to EURORDIS, and about 17,500 patients are registered with life-threatening and chronically progressive rare diseases in the Russian Federation. Additionally, the growing focus on enhancing the development of adeno associated virus vector for gene and cell therapy research, along with the increasing government biomedical research funding, is supporting the market growth. For instance, the NSW Government is building a viral vector manufacturing facility in western Sydney via A$49.6 million investment for the viral vector manufacturing facility (VVMF).
Restraining Factors
The adeno associated virus vector manufacturing market is restricted by factors like strict regulations, technical complexities, and challenges related to production & scalability. Increased operational costs related to cell & gene therapy manufacturing are challenging the adeno associated virus vector manufacturing market.
Market Segmentation
The adeno associated virus vector manufacturing market share is classified into scale of operations, therapeutic area, and application.
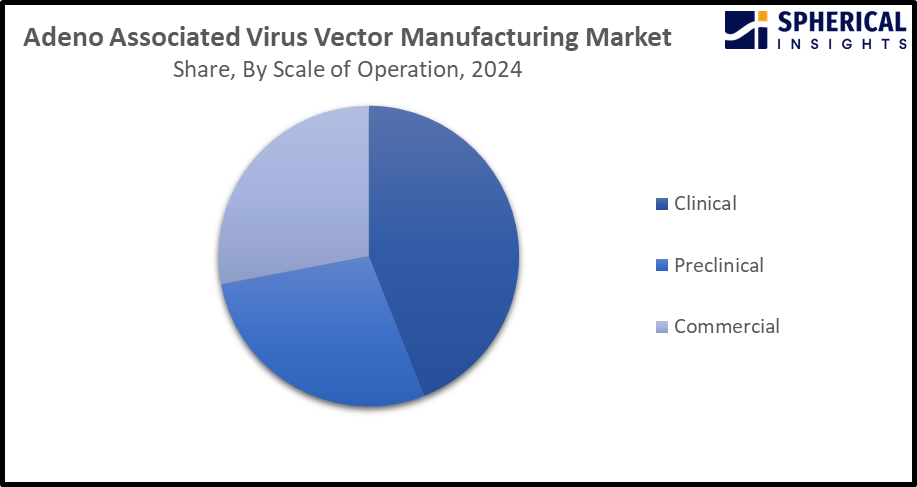
- The clinical segment dominated the market with a major share of 47.5% in 2024 and is projected to grow at a substantial CAGR during the forecast period.
Based on the scale of operations, the adeno associated virus vector manufacturing market is divided into clinical, preclinical, and commercial. Among these, the clinical segment dominated the market with a major share of 47.5% in 2024 and is projected to grow at a substantial CAGR during the forecast period. Use of rAAV is being encouraged for therapeutic gene transfer to patients in a clinical setting. Further, its use in clinical trials, driving the demand for production and purification systems for generating a large amount of highly pure rAAV particles. An increasing number of clinical trials targeting various genetic disorders is contributing to driving the market demand.
Get more details on this report -
- The neurological disorders segment dominated the adeno associated virus vector manufacturing market in 2024 and is anticipated to grow at a significant CAGR of 22.3% during the forecast period.
Based on the therapeutic area, the adeno associated virus vector manufacturing market is divided into neurological disorders, metabolic disorders, ophthalmic disorders, muscular/neuromuscular disorders, and others. Among these, the neurological disorders segment dominated the adeno associated virus vector manufacturing market in 2024 and is anticipated to grow at a significant CAGR of 22.3% during the forecast period. AAV is the most important vector for CNS gene therapy. Some of the AAV-based drugs for neurological disorders include Upstaza, Luxturna, and Zolgensma. An increasing emphasis on addressing neurodegenerative diseases and genetic neurological disorders, driving the use of AAV vectors for targeted and precise interventions, is propelling the market growth.
- The vaccine segment held the dominant market share of 57.8% in 2024 and is anticipated to grow at a significant CAGR of 21.8% during the forecast period.
Based on the application, the adeno associated virus vector manufacturing market is divided into gene therapy, vaccine, cell therapy, and others. Among these, the vaccine segment held the dominant market share of 57.8% in 2024 and is anticipated to grow at a significant CAGR of 21.8% during the forecast period. AAV vectors are potentially used for gene vaccine delivery for both infectious and non-infectious diseases like influenza, COVID-19, Alzheimer's disease, and cancer. It works by delivering nucleic acid components encoded with the target protein for establishing an immune defense. The growing expansion of vaccine development initiatives, along with advancements in vector design and production platforms, is promoting the segmental market growth.
Regional Segment Analysis of the Adeno Associated Virus Vector Manufacturing Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
North America is anticipated to hold the largest share of the adeno associated virus vector manufacturing market over the predicted timeframe.

Get more details on this report -
North America is anticipated to hold the largest share of 48.5% in the adeno associated virus vector manufacturing market over the predicted timeframe. The market ecosystem in North America is strong, with an increasing strategic alliances in the pharma and biotech industries for promoting innovation. For instance, in June 2024, ProBio Inc, a contract development and manufacturing organization (CDMO), would be growing its plasmid DNA (pDNA) and viral vector manufacturing services by opening a new 128,000 square-foot plant in Hopewell, NJ. The demand for adeno associated virus vector manufacturing has been driven by the region's advanced healthcare infrastructure and growing innovation. United States is leading the North America adeno associated virus vector manufacturing market, driven by the prevalence of target diseases and the availability of funding for gene therapy development.
Asia Pacific is expected to grow at a rapid CAGR of 22.0% in the adeno associated virus vector manufacturing market during the forecast period. The Asia Pacific area has a thriving market for adeno associated virus vector manufacturing due to cutting-edge technologies, including gene therapy advancement, vaccine development, and innovation in cancer treatments, gene editing technologies, especially CRISPR. Governments' increasing investment in the biotechnology industry, with an increasing understanding of gene therapy potential, is propelling the market growth. China is leading the Asia Pacific adeno associated virus vector manufacturing market with a 20.8% CAGR, owing to the increasing implementation of AAV vectors as promising and popular tools for genetic medication.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the adeno associated virus vector manufacturing market, along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Oxford Biomedica PLC
- GenScript (ProBio)
- Yposkesi, Inc.
- WuXi AppTec
- Sarepta Therapeutics, Inc.
- Pfizer Inc.
- Roche
- Audentes Therapeutics
- BioMarin Pharmaceutical
- F. Hoffmann-La Roche Ltd
- Charles River Laboratories
- Genezen
- Creative Biogene
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In October 2025, Asimov launched its AAV Edge Stable producer system, intending to address the shortcomings of traditional transient transfection-based production and bring AAV manufacturing standards.
- In October 2025, OXB, a global quality and innovation-led cell and gene therapy CDMO, announced that it has signed and closed an asset purchase transaction to acquire a custom built, state-of-the-art.
- In July 2025, ProBio, a global contract development and manufacturing organization (CDMO) specializing in cell and gene therapy, celebrated the opening of its facility in Hopewell, New Jersey. The facility would focus on the production of high-quality plasmid, lentiviral, and adeno-associated viral (AAV) vectors for genomic medicine.
- In August 2025, ProBio, a contract development and manufacturing organization (CDMO) specializing in gene and cell therapy, announced the launch of current good manufacturing practice (cGMP) adeno associated virus (AVV) manufacturing services at its 128,000 sq ft facility in Hopewell, N.J. cell and gene therapy viral vector manufacturing facility in North Carolina from RTP Operating, LLC, a subsidiary of National Resilience Holdco, Inc.
- In May 2025, CDMO 3PBIOVIAN officials reported that the company has launched the AAVion platform, a fully integrated adeno associated virus (AAV) manufacturing solution designed to accelerate gene therapy development.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the adeno associated virus vector manufacturing market based on the below-mentioned segments:
Global Adeno Associated Virus Vector Manufacturing Market, By Scale of Operations
- Clinical
- Preclinical
- Commercial
Global Adeno Associated Virus Vector Manufacturing Market, By Therapeutic Area
- Neurological Disorders
- Metabolic Disorders
- Ophthalmic Disorders
- Muscular/Neuromuscular Disorders
- Others
Global Adeno Associated Virus Vector Manufacturing Market, By Application
- Gene Therapy
- Vaccine
- Cell Therapy
- Others
Global Adeno Associated Virus Vector Manufacturing Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the market size of the adeno associated virus vector manufacturing market?The global adeno associated virus vector manufacturing market size is expected to grow from USD 1015.5 Million in 2024 to USD 6470.5 Million by 2035, at a CAGR of 18.34% during the forecast period 2025-2035.
-
2. Which region holds the largest share of the adeno associated virus vector manufacturing market?North America is anticipated to hold the largest share of the adeno associated virus vector manufacturing market over the predicted timeframe.
-
3. What is the forecasted CAGR of the Global Adeno Associated Virus Vector Manufacturing Market from 2024 to 2035?The market is expected to grow at a CAGR of around 18.34% during the period 2024–2035.
-
4. Who are the top companies operating in the Global Adeno Associated Virus Vector Manufacturing Market?Key players include Oxford Biomedica PLC, GenScript (ProBio), Yposkesi, Inc., WuXi AppTec, Sarepta Therapeutics, Inc., Pfizer Inc., Roche, Audentes Therapeutics, BioMarin Pharmaceutical, and F. Hoffmann-La Roche Ltd.
-
5. Can you provide company profiles for the leading adeno associated virus vector manufacturing manufacturers?Yes. For example, Oxford Biomedica PLC is a contract development and manufacturing services organization that specialized in cell and gene therapy, provides LentiVector, a lentiviral vector delivery platform and adeno-associated virus (AAV) platform to various pharmaceutical and biotechnology companies. GenScript (ProBio) is a global contract development and manufacturing organization (CDMO) specializing in cell and gene therapy.
-
6. What are the main drivers of growth in the adeno associated virus vector manufacturing market?The growing prevalence of genetic disorders, the development of adeno-associated virus vector for gene and cell therapy research, and increasing government biomedical research funding, are major market growth drivers of the adeno associated virus vector manufacturing market.
-
7. What challenges are limiting the adeno associated virus vector manufacturing market?Strict regulations, technical complexities, and increased operational costs related to cell & gene therapy manufacturing remain key restraints in the adeno associated virus vector manufacturing market.
Need help to buy this report?