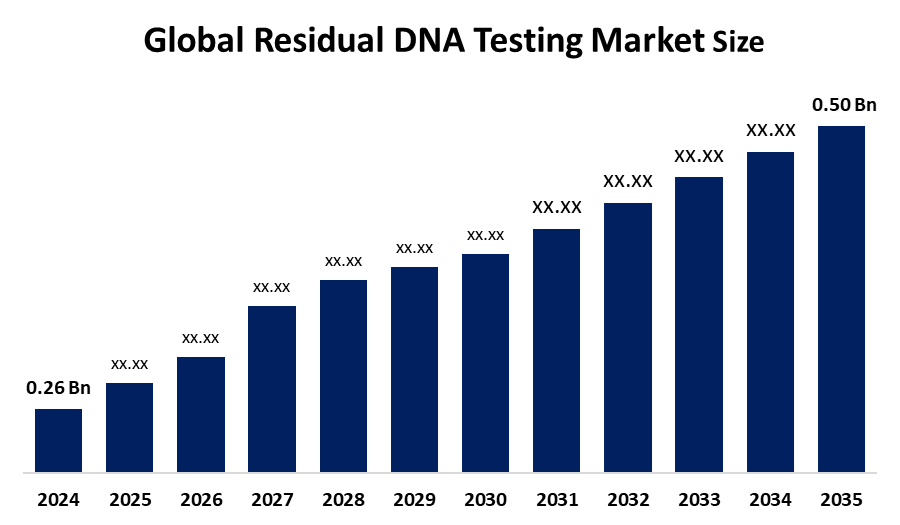
Global Residual DNA Testing Market size was valued at USD 0.50 billion by 2035| CAGR of 6.13 %
Category: HealthcareGlobal Residual DNA Testing Market Size Was Valued At USD 0.50 Billion By 2035
According to a research report published by Spherical Insights & Consulting, The Global Residual DNA Testing Market Size was Estimated to Grow from USD 0.26 Billion in 2024 to USD 0.50 Billion by 2035, at a CAGR of 6.13 % during the forecast period 2025-2035.
Get more details on this report -
Browse 210 Market Data Tables And 45 Figures Spread Through 190 Pages and In-Depth TOC On The Global Residual DNA Testing Market Size, Share, and COVID-19 Impact Analysis, By Technology (Polymerase Chain Reaction, Threshold Assays, DNA Probe Hybridization, and Others), By Application (Monoclonal Antibodies, Cell & Gene Therapy, Vaccines, and Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 – 2035. ” Detailed Report Decription Here:https://www.sphericalinsights.com/reports/residual-dna-testing-market
The global sector devoted to the creation, manufacturing, and use of analytical techniques for identifying and measuring traces of host cell DNA still present in biologic products is known as the residual DNA testing market. In the production of biologics, vaccines, gene treatments, and other biopharmaceutical products made from living cells, residual DNA testing is an essential part of quality control. In order to protect product safety, efficacy, and patient health, these tests make sure that residual DNA levels adhere to regulatory limitations set by health authorities. The market includes a range of testing technologies, such as next-generation analytical tools, hybridization tests, and polymerase chain reaction (PCR)-based techniques, along with associated chemicals, kits, and services. Strict regulations enforced by international health authorities are another important factor. Strict restrictions on the amount of residual DNA in biologic products are imposed by regulatory bodies, which forces producers to use sensitive and dependable testing techniques all the way through the production process. The need for sophisticated residual DNA testing solutions rises as a result of compliance with these standards. Technological developments in analytical methods and molecular biology also support the market. However, the market for residual DNA testing is restricted by high prices, complicated technology, a lack of experience, and difficulties with standardization.
The polymerase chain reaction segment accounted for the largest share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period.
Based on the technology, the residual DNA testing market is divided into polymerase chain reaction, threshold assays, DNA probe hybridization, and others. Among these, the polymerase chain reaction segment accounted for the largest share in 2024 and is anticipated to grow at a significant CAGR during the forecast period. Its high sensitivity, precision, and quick detection capabilities are what propel the polymerase chain reaction (PCR) market. In biopharmaceutical quality control, PCR-based techniques are frequently used to find traces of residual DNA in biologic products.
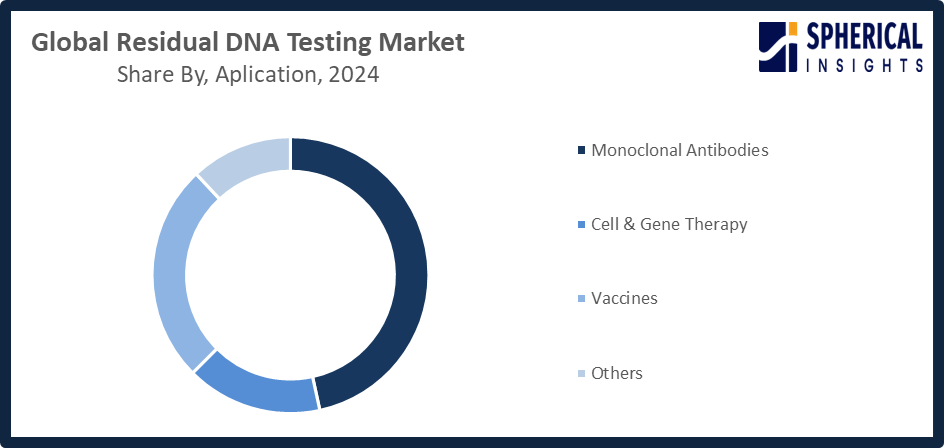
The monoclonal antibodies segment accounted for the highest market revenue in 2024 and is anticipated to grow at a significant CAGR during the forecast period.
Based on the application, the residual DNA testing market is divided into monoclonal antibodies, cell & gene therapy, vaccines, and others. Among these, the monoclonal antibodies segment accounted for the highest market revenue in 2024 and is anticipated to grow at a significant CAGR during the forecast period. The growing manufacturing of monoclonal antibody-based biologics worldwide and their extensive therapeutic use are driving the monoclonal antibodies market.
Get more details on this report -
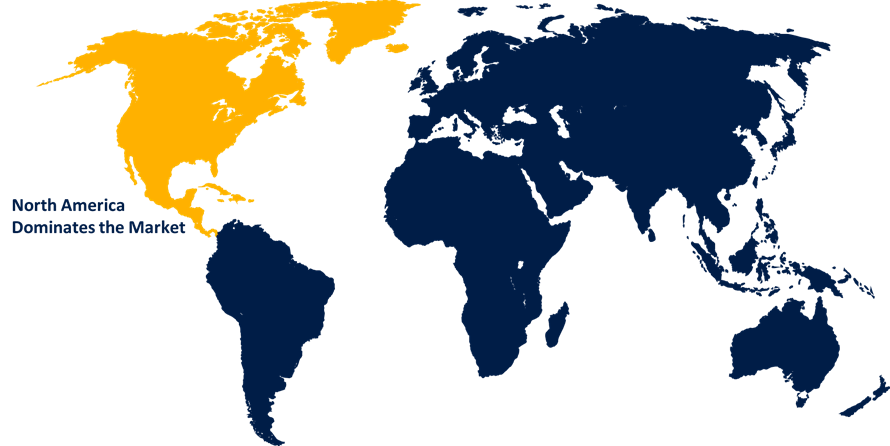
North America is expected to hold the majority share of the global residual DNA testing market during the forecast period.
Get more details on this report -
North America is expected to hold the majority share of the global residual DNA testing market during the forecast period. The demand for residual DNA testing is consistently driven by strict regulatory frameworks implemented by organizations like the U.S. Food and Drug Administration, which require biologic goods to undergo thorough quality control and safety testing. Market dominance in the area is further supported by significant investments in R&D and the early adoption of cutting-edge molecular testing technology. Furthermore, the demand for accurate and trustworthy residual DNA testing is strengthened by North America's growing production of biologics, vaccines, and cell- and gene-based treatments.
Asia Pacific is anticipated to grow at the fastest pace in the global residual DNA testing market during the forecast period. Manufacturers are being encouraged to use residual DNA testing as a normal quality control procedure due to increased awareness of product safety and regulatory compliance. Modern molecular testing technologies are becoming more widely used, and improvements in laboratory infrastructure are accelerating market expansion.
Major vendors in the global residual DNA testing market are Bio-Rad Laboratories, Inc., Charles River Laboratories, Eurofins Scientific, F. Hoffmann-La Roche Ltd., FUJIFILM Corporation, Intertek Group plc, Jiagnsu Hillgene Biopharma Co., Ltd., Lonza, Maravai LifeSciences, Merck KGaA, QIAGEN, Sartorius AG, Thermo Fisher Scientific Inc., WuXi AppTec, and Others.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In September 2024, after acquiring Infinity Laboratories, Eurofins Scientific increased BioPharma product testing services in the US, improving its capacity for residual DNA testing and biologics quality assurance.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the residual DNA testing market based on the below-mentioned segments:
Global Residual DNA Testing Market, By Technology
- Polymerase Chain Reaction
- Threshold Assays
- DNA Probe Hybridization
- Others
Global Residual DNA Testing Market, By Application
- Monoclonal Antibodies
- Cell & Gene Therapy
- Vaccines
- Others
Global Residual DNA Testing Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Follow Us: LinkedIn | Facebook | Twitter
Need help to buy this report?