Global Oncology Companion Diagnostic Market Size to Exceed USD 13.38 Billion by 2035 | CAGR of 9.13%
Category: HealthcareGlobal Oncology Companion Diagnostic Market Size to Exceed USD 13.38 Billion by 2035
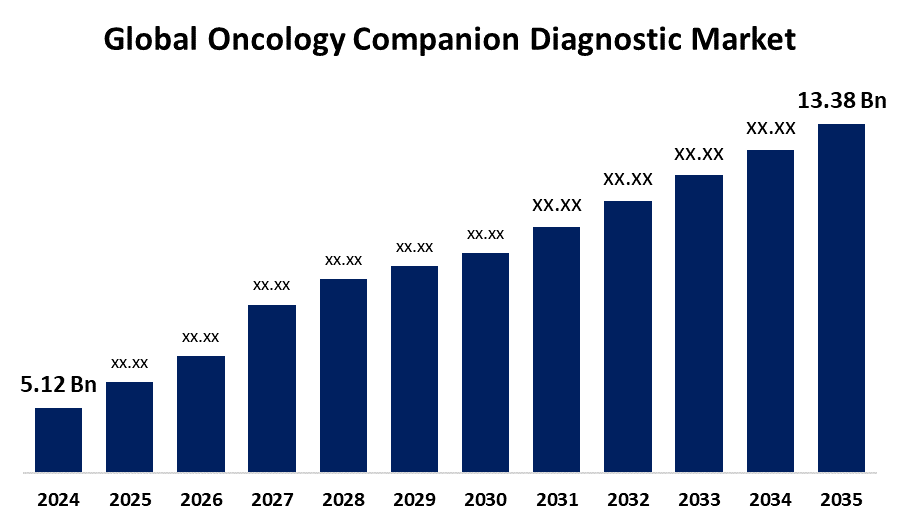
According to a Research Report Published by Spherical Insights & Consulting, The Global Oncology Companion Diagnostic Market Size is expected to Grow from USD 5.12 Billion in 2024 to USD 13.38 Billion by 2035, at a CAGR of 9.13% during the forecast period 2025-2035.
Get more details on this report -
Browse 210 Market Data Tables And 45 Figures Spread Through 190 Pages and In-Depth TOC On the "Global Oncology Companion Diagnostic Market Size, Share, and COVID-19 Impact Analysis, By Technology (Polymerase Chain Reaction (PCR), Next-generation Sequencing (NGS), Immunohistochemistry (IHC), In Situ Hybridization (ISH)/Fluorescence In Situ Hybridization (FISH), and Others), By End Use (Hospital, Pathology/Diagnostic Laboratory, and Academic Medical Center), By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 - 2035." Get Detailed Report Description Here: https://www.sphericalinsights.com/reports/oncology-companion-diagnostic-market
The oncology companion diagnostic market includes tests that identify specific genetic or molecular markers in cancer patients, guiding the selection of effective targeted therapies to improve treatment outcomes and reduce unnecessary interventions. The rising prevalence of cancer and the expanding usage of targeted medicines, which call for accurate diagnostic tools, are driving the oncology companion diagnostic market's rapid expansion. In vitro diagnostic tests known as companion diagnostics offer crucial data for the safe and efficient administration of related cancer treatments. Advances in AI, machine learning, and genomic technologies benefit the market by improving the precision and effectiveness of diagnosis. In order to better match treatments to individual cancer profiles, research and development efforts are increasingly concentrated on creating novel biomarkers and multi-gene panels. Important advancements include Guardant Health and Boehringer Ingelheim's December 2024 partnership to promote HER2-targeted liquid biopsies and the FDA's August 2024 approval of Illumina's TruSight Oncology Comprehensive test. These developments are speeding up innovation, regulatory approval, and the global use of precision oncology techniques, along with international collaborations between academic institutions and healthcare organisations. Early, more individualised, and more economical treatment approaches are made possible by these changes, which are revolutionising cancer care. For technologies like NGS, high R&D expenses and little insurance coverage impede smaller businesses and lower market penetration overall.
The polymerase chain reaction (PCR) segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period.
Based on the technology, the oncology companion diagnostic market is divided into polymerase chain reaction (PCR), next-generation sequencing (NGS), immunohistochemistry (IHC), in situ hybridization (ISH)/fluorescence in situ hybridization (FISH), and others. Among these, the polymerase chain reaction (PCR) segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period. PCR is a widely utilised technology in the oncology companion diagnostics market. Its high sensitivity and selectivity make it a preferred approach for detecting genetic alterations and cancer biomarkers, which have an impact on the growth of the oncology companion diagnostics market. Finding tumour-specific alterations or variations in a patient's tumour helps determine the best course of action.
The hospital segment accounted for the fastest share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period.
Based on the end use, the oncology companion diagnostic market is divided into hospital, pathology/diagnostic laboratory, and academic medical center. Among these, the hospital segment accounted for the largest share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. The hospitals sector dominated the oncology companion diagnostic market in 2024 because of their specialist molecular diagnostic labs, polymathic cancer care teams, and systematic laboratory setups that allow for the integration of testing into treatment plans.
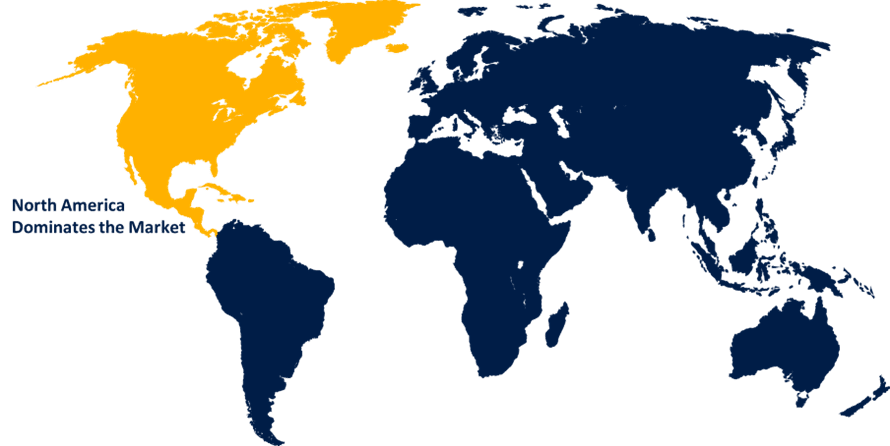
North America is expected to hold the majority share of the global oncology companion diagnostic market during the forecast period.
Get more details on this report -
North America is expected to hold the majority share of the global oncology companion diagnostic market during the forecast period. The sophisticated, well-established healthcare infrastructure is propelling the market's expansion by encouraging the purchase of cutting-edge diagnostic equipment. Furthermore, the extensive range of uses of diagnostic technologies, including companion diagnostics, that are required for individualised cancer treatment, is made possible by significant investments in healthcare.
Asia Pacific is anticipated to grow at the fastest pace in the global oncology companion diagnostic market during the forecast period. The increased industrialisation and urbanisation throughout the region. Driven by a growing population, improved infrastructure, healthcare reforms, and the entry of more local businesses into the market. The area is a major focus for oncology developments due to its large population and high cancer frequency.
Major vendors in the global oncology companion diagnostic market are Agilent Technologies, Inc., Illumina, Inc., QIAGEN, Thermo Fisher Scientific Inc., Foundation Medicine, Inc., Myriad Genetics, Inc., F. Hoffmann-La Roche Ltd, BIOMÉRIEUX, Abbott, Leica Biosystems Nussloch GmbH, Guardant Health, EntroGen, Inc., and Others.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In January 2025, FoundationOne CDx was approved by the FDA as the first and only companion diagnostic for OJEMDA (tovorafenib), a technology II RAF inhibitor, to treat paediatric patients aged six months and up with recurrent or refractory BRAF-altered low-grade glioma (pLGG). This approval allows clinicians to detect patients with BRAF fusions, rearrangements, or BRAF V600 mutations in their tumours, allowing for more targeted treatment with tovorafenib. Previously, no FDA-approved therapies were available for tumours with BRAF fusions, which account for around 80% of BRAF-altered pLGG cases.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the oncology companion diagnostic market based on the below-mentioned segments:
Global Oncology Companion Diagnostic Market, By Technology
- Polymerase Chain Reaction (PCR)
- Next-generation Sequencing (NGS)
- Immunohistochemistry (IHC)
- In Situ Hybridization (ISH)/Fluorescence in Situ Hybridization (FISH)
- Others
Global Oncology Companion Diagnostic Market, By End Use
- Hospital
- Pathology/Diagnostic Laboratory
- Academic Medical Center
Global Oncology Companion Diagnostic Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Need help to buy this report?