Global Biologics Safety Testing Market Size To Exceed USD 13.0 Billion By 2035 | CAGR Of 11.06%
Category: HealthcareGlobal Biologics Safety Testing Market Size To Exceed USD 13.0 Billion By 2035
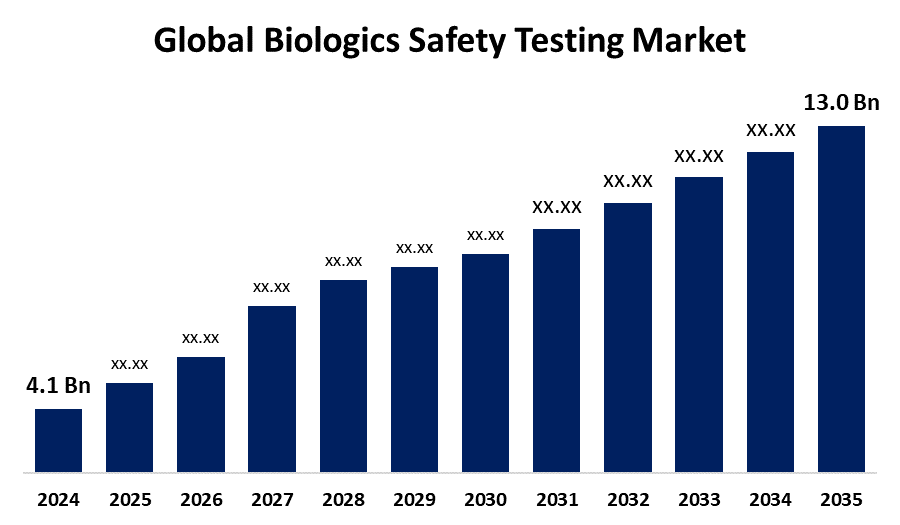
According to a research report published by Spherical Insights & Consulting, The Global Biologics Safety Testing Market Size is expected to Grow from USD 4.1 Billion in 2024 to USD 13.0 Billion by 2035, at a CAGR of 11.06% during the forecast period 2025-2035.
Get more details on this report -
Browse key industry insights spread across 250 pages with 100 Market data tables and figures & charts from the report on the “Global Biologics Safety Testing Market Size, Share, and COVID-19 Impact Analysis, By Test Type (Mycoplasma, Sterility, Endotoxin, Bioburden, and Virus Safety), By Application (Vaccines & Therapeutics, Blood & Blood Products, Tissue & Tissue Products, Cellular & Gene Therapy Products, and Stem Cell Products), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 – 2035.” Get Detailed Report Description Here: https://www.sphericalinsights.com/reports/biologics-safety-testing-market
The biologics safety testing market is the industry of testing and characterization of biological products for ensuring their safety and efficiency, especially for products like vaccines, gene therapies, and biopharmaceuticals. Biologics safety testing services aid in verifying the absence of bacterial contaminants, thereby ensuring safety of vaccines and biopharmaceuticals. Innovative approaches and advanced technologies in the next generation therapeutics by various biotechnology and pharmaceutical organizations for improved efficacy, precision, and safety profiles are escalating market growth opportunities for biologics safety testing. The development of monoclonal antibodies (mAbs) and biosimilars, driving the need for safety testing to ensure patient safety and product quality, is propelling the biologics safety testing market demand. However, an increased cost and high capital investment required for safety testing in the development of biologics drugs are challenging the market.
The endotoxin segment dominated the market with a significant market share in 2024 and is projected to grow at a substantial CAGR during the forecast period.
Based on the test type, the biologics safety testing market is divided into mycoplasma, sterility, endotoxin, bioburden, and virus safety. Among these, the endotoxin segment dominated the market with a significant market share in 2024 and is projected to grow at a substantial CAGR during the forecast period. The use of endotoxin safety testing for ensuring the safety of pharmaceuticals and other medical device products, especially intravenous and injectable products are driving the market in the endotoxin segment.
The vaccines & therapeutics segment accounted for a substantial market share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period.
Based on the application, the biologics safety testing market is divided into vaccines & therapeutics, blood & blood products, tissue & tissue products, cellular & gene therapy products, and stem cell products. Among these, the vaccines & therapeutics segment accounted for a substantial market share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. The use of biologics safety testing for ensuring vaccine efficacy and therapeutics, as well as strict regulations emphasizing the safety and efficacy of vaccines, is propelling the market.
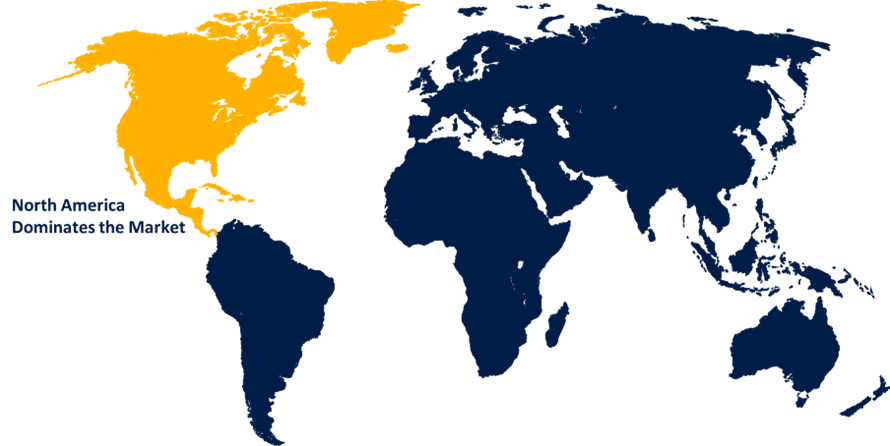
North America is expected to hold the majority share of the global biologics safety testing market during the forecast period.
Get more details on this report -
North America is expected to hold the majority share of the global biologics safety testing market during the forecast period. The growing demand for biologics products, including mAb, vaccines, and cell treatments, due to the prevalence of chronic diseases, is contributing to drive the market demand. The demand for extensive safety assessments due to the enforcement of stringent safety standards by the USFDA and other regulatory bodies is driving the market demand.
Asia Pacific is anticipated to grow at the fastest pace in the global Biologics Safety Testing market during the forecast period. The increasing need for biologics and biosimilars, along with increasing R&D activities for advancing the biotechnology industry, are propelling the market. The growing biopharmaceutical investment and R&D activities for advancing biotechnology industry are driving regional market.
Europe is anticipated to hold a substantial market share of the biologics safety testing market during the predicted timeframe. The growing need for safe and effective biologics with the expanding biopharmaceutical industry is driving the market demand. The presence of well-established healthcare infrastructure also responsible for propelling the regional market growth.
Major vendors in the global biologics safety testing market are Abcam, PathogenDx, Thermo Fisher Scientific, Boehringer Ingelheim, Vancouver Testing Laboratories, Sartorius AG, Wuxi AppTec, Lonza Group, Merck KGaA, SGS SA, Charles River Laboratories, Invetech, BioReliance, Eurofins Scientific, WuXi PharmaTech, and Others.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Key Market Development
- In January 2024, Charles River Laboratories International has advanced its flagship Endosafe cartridge technology and combined it with its recombinant cascade reagent (rCR) to launch the Endosafe Trillium rCR cartridge offering.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the biologics safety testing market based on the below-mentioned segments:
Global Biologics Safety Testing Market, By Test Type
- Mycoplasma
- Sterility
- Endotoxin
- Bioburden
- Virus Safety
Global Biologics Safety Testing Market, By Application
- Vaccines & Therapeutics
- Blood & Blood Products
- Tissue & Tissue Products
- Cellular & Gene Therapy Products
- Stem Cell Products
Global Biologics Safety Testing Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Need help to buy this report?