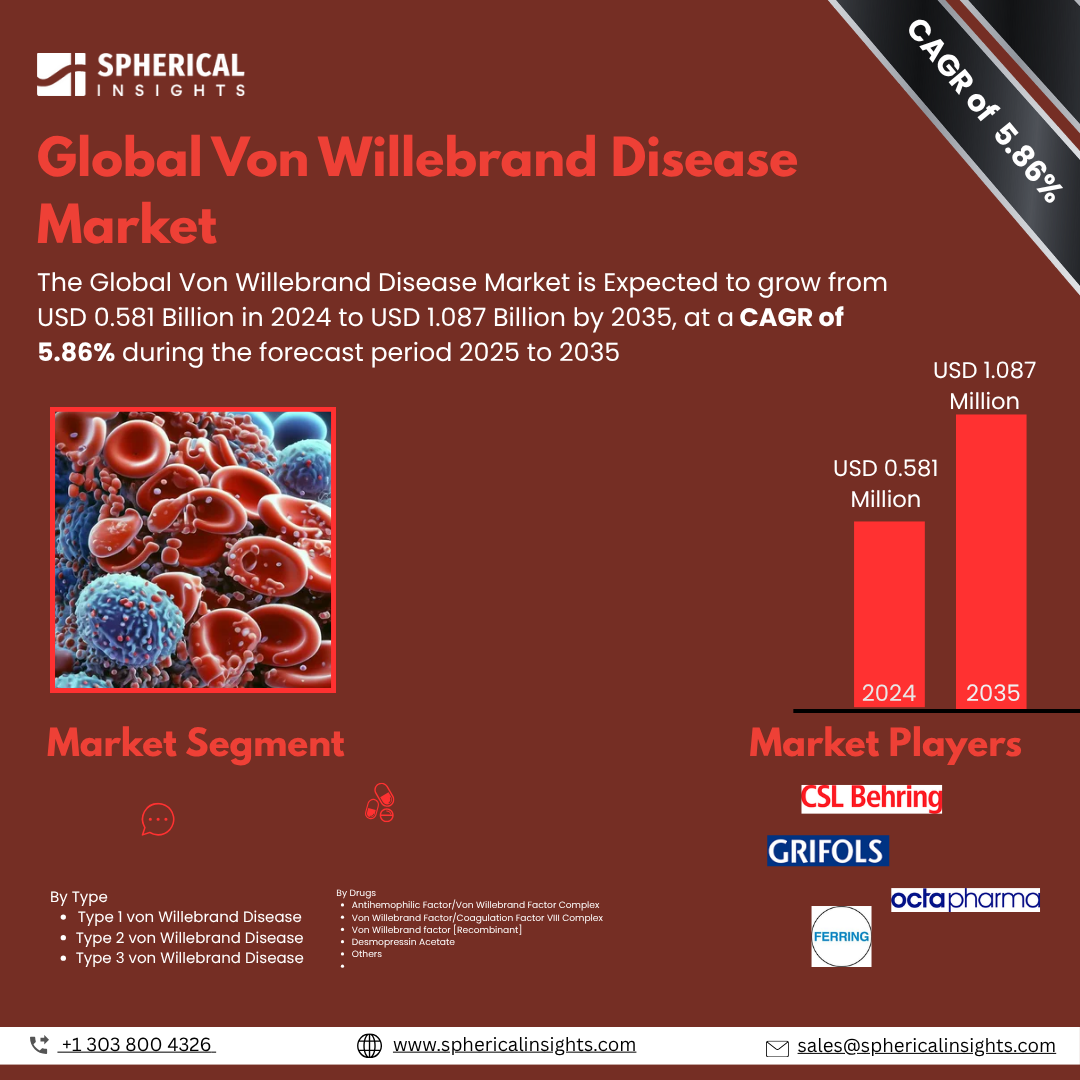
- As per Spherical Insights & Consulting, The Global Von Willebrand Disease Market Size is Expected to Grow from USD 0.581 Billion in 2024 to USD 1.087 Billion by 2035, at a CAGR of 5.86% during the forecast period 2025 2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Von Willebrand Disease Market Companies such as CSL Behring, Grifols, Octapharma, Bio Products Laboratory, Takeda (Shire), Sanofi, Pfizer, Ferring Pharmaceuticals, Bayer, Novo Nordisk, Kedrion Biopharma, LFB Group, ADMA Biologics, CSL Vifor, Aptevo Therapeutics, and Others.
Von Willebrand Disease Treatment Market: Understanding and Treatment Algorithm:
Von Willebrand Disease (VWD) is a genetic bleeding disorder caused by a deficiency or dysfunction of von Willebrand factor, a protein essential for blood clotting. It leads to prolonged bleeding, frequent nosebleeds, easy bruising, and heavy menstrual flow. VWD is the most common inherited bleeding disorder and varies in severity.
Von Willebrand Disease Diagnosis:
Diagnosis of Von Willebrand Disease involves a combination of blood tests to measure von Willebrand factor levels, activity, and associated clotting factors like factor VIII. Tests may include VWF antigen, ristocetin cofactor activity, and platelet function. Accurate diagnosis requires specialized labs, especially since symptoms overlap with other bleeding disorders.
Von Willebrand Disease Treatment
Treatment of Von Willebrand Disease depends on its type and severity. Common approaches include desmopressin to boost natural VWF levels and replacement therapies using VWF concentrates for more severe cases. Other treatments may involve antifibrinolytics and hormonal therapies. Management aims to prevent or control bleeding during surgeries, injuries, or menstruation.
Von Willebrand Disease Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Von Willebrand Disease, Gender-specific Diagnosed Incidence of Von Willebrand Disease, Type specific Diagnosed Incidence of Von Willebrand Disease, Age specific Diagnosed Incidence of Von Willebrand Disease, Diagnosed Incident Population based on Primary Site of Von Willebrand Disease, and Diagnosed Incident Population based on Histologic Classification of Von Willebrand Disease Tumour in the global market covering North America, Europe, Asia Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of Von Willebrand Disease epidemiology in major markets worldwide.
Country Wise- Von Willebrand Disease Multiforme Epidemiology
The epidemiology segment provides Von Willebrand Disease prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa
Von Willebrand Disease: Recent Development:
• In January 2022, Takeda announced that the FDA had approved VONVENDI (recombinant non Willebrand factor) for routine prophylaxis to reduce bleeding episodes in adult with severe type 3 VMD. This marked the first recombinant VWF therapy authorized for ongoing prophylactic use, following data showing a 54.7% median reduction in annualized bleeding rate
Von Willebrand Disease Marketed Drugs:
VONVENDI (recombinant von Willebrand factor) is the first FDA approved recombinant treatment for Von Willebrand Disease. It is used for on-demand treatment and control of bleeding episodes, perioperative management, and routine prophylaxis in adults with severe type 3 VWD. It replaces the deficient or defective VWF to aid normal blood clotting.
WILATE is a plasma-derived, double virus inactivated von Willebrand factor and factor VIII concentrate. Approved for on demand treatment, surgical prophylaxis, and routine prevention of bleeding episodes in VWD patients. Its VWF to FVIII ratio closely resembles that of human plasma, providing balanced hemostatic support with high viral safety.
HUMATE P is a plasma derived concentrate containing von Willebrand factor and factor VIII, used to treat and prevent bleeding in VWD, especially during surgery. It is one of the most widely used therapies for VWD management, with proven efficacy in controlling major bleeding episodes across various VWD types.
Von Willebrand Disease: Emerging Therapies
BT200: It is pegylated aptamer that binds tha VMF A1 domain, prolonging VMF and FVIII half life. In phase !! trais for type 2B VWD, it more than doubled VWF FVIII levels and tripled platelet counts in thrombocytopenic patients,
Phase II data showed a 75-88% reduction in annunalized bleeding rates, and it recived FDA Fast Track designation
HMB 002: It is a first class antibody administered subcutaneously , stabilizing endogenous VWF. The phase 1 and 2 VELORA Pioneer study showed cunta a rise in vmf within 14 days and normalized coagulation metrics, with no serious adverse events.
Von Willebrand Disease Market Outlook
- The Von Willebrand Disease market comprises diagnostic tools, treatments, and therapeutics addressing the inherited bleeding disorder caused by von Willebrand factor deficiency or dysfunction. This market includes recombinant therapies, plasma-derived products, and supportive care, aiming to manage bleeding symptoms across various VWD types and severities globally.
- Key drivers include rising awareness of bleeding disorders, improved diagnostic techniques, and advancements in recombinant and long-acting factor therapies. Increased healthcare expenditure, robust R&D investment, and growing patient registries also contribute to expanding treatment accessibility and accelerating the adoption of novel therapeutic solutions for Von Willebrand Disease.
- Opportunities lie in developing subcutaneous and gene therapies, enhancing early diagnosis through genetic screening, and increasing patient education. Emerging markets offer untapped growth due to improving healthcare infrastructure and access. Strategic partnerships and personalized treatment approaches also open new commercial and clinical pathways in this space.
- Governments worldwide have promoted rare disease frameworks, subsidized VWD treatments, and expanded national hemophilia programs. Support includes increased funding for bleeding disorder research, awareness campaigns, and implementation of newborn screening and prophylaxis programs, especially in developed and emerging economies, prioritizing rare disease management.
- Limited diagnosis in mild or asymptomatic cases remains a key barrier.
- Projected growth is driven by increasing use of prophylactic and recombinant therapies in both developed and developing regions.
Von Willebrand Disease Market Segmentation
By Treatment Type:
- Type 1 Von Willebrand Disease
- Type 2 Von Willebrand Disease
- Type 3 Von Willebrand Disease
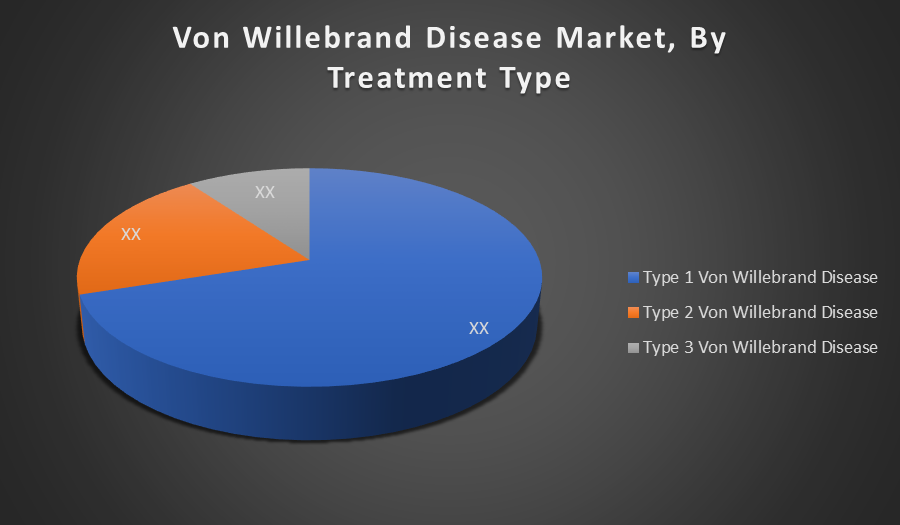
Type 1 Von Willebrand Disease holds the largest market share due to its high prevalence, representing over 70% of all VWD cases. It is typically mild and manageable with first-line treatments like desmopressin, leading to greater diagnosis rates and routine treatment, which significantly boosts its share in the global market.
By Drugs:
- Antihemophilic Factor/Von Willebrand Factor Complex
- Von Willebrand Factor/Coagulation Factor VIII Complex
- Von Willebrand factor [Recombinant]
- Desmopressin Acetate
- Others
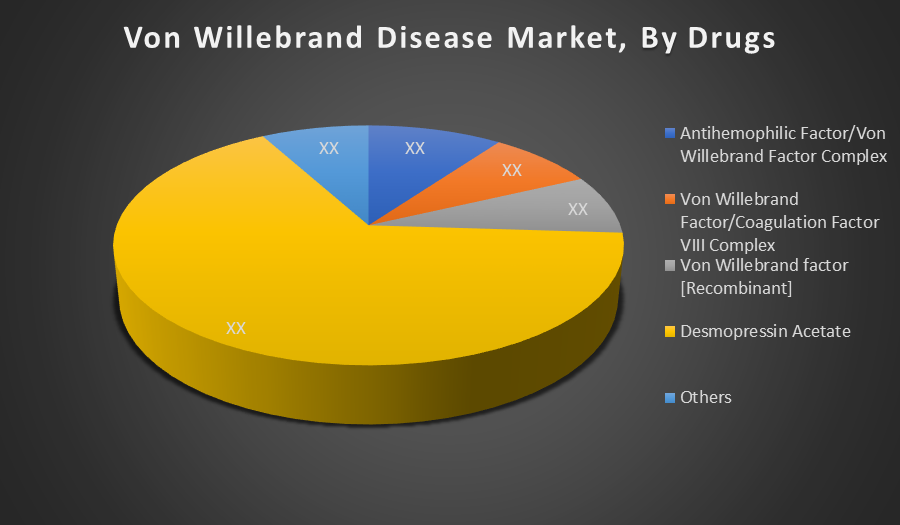
Desmopressin Acetate dominates the drug segment because it is the standard first line treatment, especially for Type 1 VWD. Its cost-effectiveness, ease of administration, and favourable safety profile make it widely used. Additionally, its effectiveness in elevating endogenous factor levels contributes to its high adoption and leading market position
Regional Segment Analysis of the Von Willebrand Disease Market
North America dominated the Von Willebrand Disease treatment market, thanks to its robust healthcare infrastructure, high awareness, and strong patient support systems. The region is driven by advance diagnostic capabilities, coordinated screening programs, and active advocacy organization. It benefits from innovation in therapies, favourable reimbursement frameworks, and a concentration of leading pharmaceutical players advancing research and development. These factors collectively position in this place
Von Willebrand Disease Market Key Companies
- CSL Behring
- Grifols
- Octapharma
- Bio Products Laboratory
- Takeda (Shire)
- Sanofi
- Pfizer
- Ferring Pharmaceuticals
- Bayer
- Novo Nordisk
- Kedrion Biopharma
- LFB Group
- ADMA Biologics
- CSL Vifor
- Aptevo Therapeutics
- Others
Von Willebrand Disease Therapeutics Market Report Scope
- The Von Willebrand Disease therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Von Willebrand Disease’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Von Willebrand Disease therapies is provided, including an evaluation of new treatments expected to influence the current Von Willebrand Disease treatment market landscape.
- The report includes a detailed review of the Von Willebrand Disease therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient-Based Von Willebrand Disease Market Forecasting report offers valuable insights into trends shaping the global Von Willebrand Disease market, helping to develop effective business strategies.
Von Willebrand Disease Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Von Willebrand Disease Therapeutic Approaches in Von Willebrand Disease
- Review Of Drugs in Development for Von Willebrand Disease
- Market, Growth, and Trends in Von Willebrand Disease
- Market Opportunities in Von Willebrand Disease Treatment
- Effects Of Future Therapies on Von Willebrand Disease Treatment.
Von Willebrand Disease Treatment Market Report Key Strengths
- 15 Years Von Willebrand Disease Market Forecast
- Global Coverage
- Von Willebrand Disease Epidemiology Segmentation
- Key Cross Competition
Von Willebrand Disease Treatment Market Report Assessment
- Present Practices in the Von Willebrand Disease Treatment Market
- Review of Investigational Von Willebrand Disease Drugs
- Attractiveness of the Von Willebrand Disease Drug Market
- Von Willebrand Disease Market Drivers
- Von Willebrand Disease Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Von Willebrand Disease market based on the below-mentioned segments:
Global Von Willebrand Disease Market, By Type
- Type 1 von Willebrand Disease
- Type 2 von Willebrand Disease
- Type 3 von Willebrand Disease
Global Von Willebrand Disease Market, By Drugs
- Antihemophilic Factor/Von Willebrand Factor Complex
- Von Willebrand Factor/Coagulation Factor VIII Complex
- Von Willebrand factor [Recombinant]
- Desmopressin Acetate
- Others
Global Von Willebrand Disease Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa