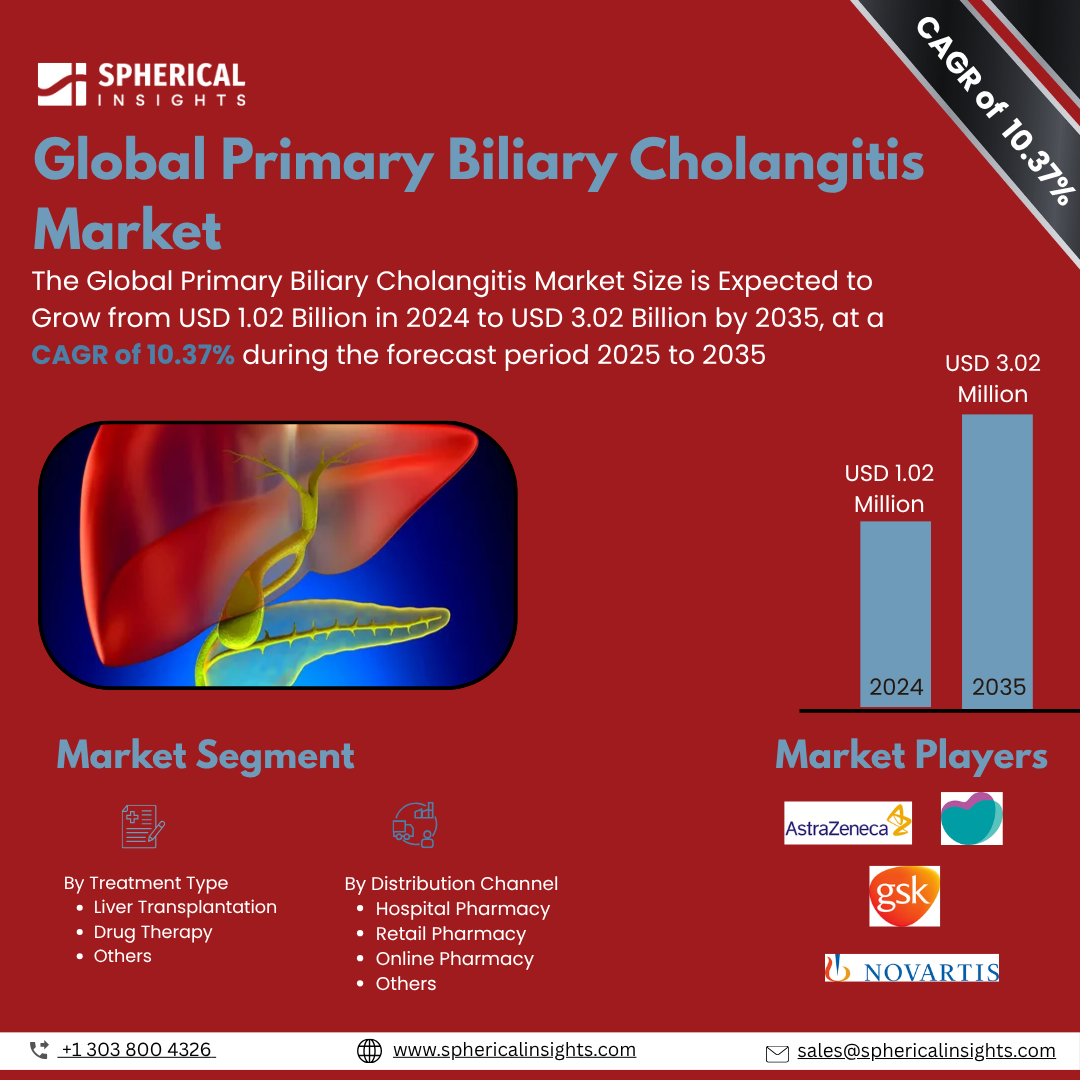
- As per Spherical Insights & Consulting, The Global Primary Biliary Cholangitis Market Size is Expected to Grow from USD 1.02 Billion in 2024 to USD 3.02 Billion by 2035, at a CAGR of 10.37% during the forecast period 2025 to 2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Primary Biliary Cholangitis Market Companies such as AstraZeneca, Zydus Lifesciences, Intercept Pharmaceuticals, GlaxoSmithKline, Mitsubishi Tanabe Pharma, Bristol Myers Squibb, Dr. Falk Pharma, AbbVie, Novartis, Johnson & Johnson, Merck & Co., Gilead Sciences, Lupin Pharmaceuticals, CymaBay Therapeutics, Genfit, and Others.
Primary Biliary Cholangitis Treatment Market: Understanding and Treatment Algorithm:
Primary Biliary Cholangitis (PBC) is a chronic autoimmune liver disease in which the body’s immune system gradually destroys the small bile ducts within the liver. This leads to bile buildup, causing liver inflammation and damage. Over time, it may progress to cirrhosis, liver failure, and the need for transplantation.
Primary Biliary Cholangitis Diagnosis:
Diagnosis of PBC typically involves blood tests showing elevated alkaline phosphatase and the presence of antimitochondrial antibodies (AMA). Liver function tests, imaging such as ultrasound or elastography, and sometimes liver biopsy confirm the disease and assess its severity. Early diagnosis allows timely treatment, which can slow or halt disease progression.
Primary Biliary Cholangitis Treatment:
Treatment of PBC focuses on slowing disease progression and relieving symptoms. Ursodeoxycholic acid is the first line therapy, while obeticholic acid is used in patients not responding to it. Other treatments manage symptoms like itching and fatigue. In advanced cases with liver failure, liver transplantation may be considered.
Primary Biliary Cholangitis Epidemiology:
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Primary Biliary Cholangitis, Gender specific Diagnosed Incidence of Primary Biliary Cholangitis, Type specific Diagnosed Incidence of Primary Biliary Cholangitis, Age specific Diagnosed Incidence of Primary Biliary Cholangitis, Diagnosed Incident Population based on Primary Site of Primary Biliary Cholangitis, and Diagnosed Incident Population based on Histologic Classification of Primary Biliary Cholangitis Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights:
This section offers a global overview of Primary Biliary Cholangitis epidemiology in major markets worldwide.
Country Wise Primary Biliary Cholangitis Multiforme Epidemiology:
- The epidemiology segment provides Primary Biliary Cholangitis prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
Primary Biliary Cholangitis: Recent Developments:
- In August 2024, Gilead Sciences announced that its drug Livdelzi (seladelpar) received accelerated approval from the US FDA for treating Primary Biliary Cholangitis. The approval was based on clinical trial data showing significant improvement in liver function and symptom relief. Gilead committed to further confirmatory trials for continued approval.
Primary Biliary Cholangitis Marketed Drugs:
• Ursodeoxycholic Acid (UDCA): Multiple Manufacturers
Ursodeoxycholic acid is a bile acid therapy and the first-line treatment for Primary Biliary Cholangitis. It works by improving bile flow and reducing liver enzyme levels, helping delay disease progression. Widely approved globally, it is most effective in early stage PBC and remains a cornerstone in long-term disease management.
• Ocaliva: Intercept Pharmaceuticals
Ocaliva (obeticholic acid) is a farnesoid X receptor (FXR) agonist used in combination with UDCA or as monotherapy in patients with inadequate UDCA response. It reduces bile acid production in the liver, improving biochemical markers of liver function. Ocaliva is FDA approved for PBC and offers an option for treatment resistant patients.
Primary Biliary Cholangitis: Emerging Therapies:
- LUM001: It is an experimental ileal bile acid transporter (IBAT) inhibitor being developed for Primary Biliary Cholangitis. It works by reducing bile acid reabsorption in the intestine, thereby lowering toxic bile acid levels in the liver. This therapy aims to improve symptoms such as pruritus and slow disease progression in PBC patients.
- Seladelpar: It is a selective peroxisome proliferator activated receptor delta (PPARδ) agonist in advanced clinical trials for PBC. It is designed to reduce liver inflammation, improve biochemical markers, and enhance quality of life. Seladelpar offers a potential treatment option for patients who are unresponsive or intolerant to standard first line therapies like UDCA.
Primary Biliary Cholangitis Market Outlook:
- The Primary Biliary Cholangitis market encompasses treatments and diagnostics aimed at managing a chronic autoimmune liver disease that progressively damages bile ducts. It includes drug therapies, transplantation, and supportive care, distributed through various healthcare channels to improve liver function and delay disease progression in affected individuals.
- Key drivers include increasing prevalence of autoimmune liver diseases, early diagnosis due to improved screening methods, and growing use of first- and second-line therapies. Advancements in drug development, strong clinical pipelines, and rising awareness among healthcare professionals also contribute to market expansion across developed and developing regions.
- Opportunities lie in the development of novel therapeutics for non-responders to standard treatment, expansion into emerging markets, and increasing research into combination therapies. Growing investment in rare disease treatment and personalized medicine approaches presents potential for improved patient outcomes and commercial success in previously underserved populations.
- Governments are supporting the PBC market through orphan drug incentives, accelerated regulatory pathways, funding for rare disease research, and public health campaigns. These initiatives aim to promote earlier diagnosis, enhance treatment access, and encourage pharmaceutical innovation in chronic autoimmune liver conditions like PBC.
- Lack of curative therapies and patient resistance to existing treatments limit long-term disease control.
- The market is expected to grow steadily due to increased adoption of advanced drug therapies and improved diagnosis rates globally.
Primary Biliary Cholangitis Market Segmentation:
By Treatment Type:
- Liver Transplantation
- Drug Therapy
- Others
Drug Therapy holds the largest market share in the treatment segment due to its effectiveness in slowing disease progression, especially in early stage PBC. First line therapies like ursodeoxycholic acid (UDCA) and obeticholic acid are widely prescribed. Their non invasive nature, long term usability, and clinical effectiveness drive dominant adoption over liver transplantation.
By Distribution Channel:
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Hospital Pharmacies account for the largest share due to the prescription-driven nature of PBC treatments and the frequent monitoring required. Patients with moderate to advanced disease stages often receive care through specialized hepatology centers within hospitals, making this channel the most reliable and secure point for accessing approved and regulated therapies.
Regional Segment Analysis of the Primary Biliary Cholangitis Market:
North America holds the largest share of the Primary Biliary Cholangitis market due to advanced healthcare infrastructure, widespread access to diagnostics, and high awareness among both patients and physicians. The presence of leading pharmaceutical companies and the availability of approved therapies such as ursodeoxycholic acid and obeticholic acid support robust treatment uptake. Additionally, favourable reimbursement policies and a structured rare disease management system contribute significantly to its market dominance.
Asia Pacific is the fastest growing region in the Primary Biliary Cholangitis market due to increasing awareness, expanding healthcare access, and rising diagnostic capabilities across countries like China, Japan, and India. Government initiatives promoting rare disease treatment, growing investment in healthcare infrastructure, and improving patient access to novel therapies are driving accelerated growth. Additionally, pharmaceutical companies are targeting this region for clinical trials and commercialization, further boosting market expansion.
Primary Biliary Cholangitis Market Key Companies:
- AstraZeneca
- Zydus Lifesciences
- Intercept Pharmaceuticals
- GlaxoSmithKline
- Mitsubishi Tanabe Pharma
- Bristol Myers Squibb
- Dr. Falk Pharma
- AbbVie
- Novartis
- Johnson & Johnson
- Merck & Co.
- Gilead Sciences
- Lupin Pharmaceuticals
- CymaBay Therapeutics
- Genfit
- Others
Market Segment:
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Primary Biliary Cholangitis market based on the following segments:
Global Primary Biliary Cholangitis Market, By Treatment Type
- Liver Transplantation
- Drug Therapy
- Others
Global Primary Biliary Cholangitis Market, By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Global Primary Biliary Cholangitis Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa